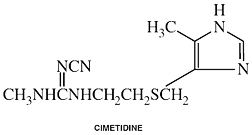

Cimetidine
Brand Names: Tagamet
Please read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
Abernethy DR, Greenblatt DJ, Divoll M, Moschitto LJ, Harmatz JS, Shader RI. Interaction of cimetidine with the triazolobenzodiazepines alprazolam and
triazolam. Psychopharmacology (Berl) 1983;80(3):275-278.
Anonymous. Cimetidine inhibits the hepatic hydroxylation of vitamin D. Nutr Rev 1985;43(6):184-185. (Review)
Bengoa JM, Bolt MJ, Rosenberg IH. Hepatic vitamin D 25-hydroxylase inhibition by cimetidine and
isoniazid. J Lab Clin Med. 1984 Oct;104(4):546-52.
Abstract: Cimetidine interaction with cytochrome P-450 may reduce binding affinity of some drugs, causing reduced metabolism of substrates such as diazepam and warfarin. Isoniazid also inhibits hepatic mixed function oxidase activity and has been reported to depress circulatory levels of hydroxylated vitamin D metabolites. Because hepatic vitamin D 25-hydroxylase is considered to be a cytochrome P-450-dependent enzyme, we examined the effect of cimetidine and isoniazid on hepatic vitamin D 25-hydroxylase activity in vitro in the rat. The assay system employed whole liver homogenates from rats deficient in vitamin D and chromatographic separation of radiolabeled substrate and product. Cimetidine and isoniazid were incubated with liver homogenates prior to the addition of 12 nmol/L final concentration of tritiated vitamin D. In addition, assays were performed in rats deficient in vitamin D given cimetidine or isoniazid 1 hour before sacrifice. Both cimetidine and isoniazid inhibited vitamin D 25-hydroxylase activity in vitro. Vitamin D 25-hydroxylase activity was depressed to 75%, 65%, and 55% of control values by cimetidine at 3, 6, and 10 mmol/L concentrations, respectively, and to 81%, 53%, and 35% by isoniazid at 1, 5, and 10 mmol/L. In vivo intraperitoneal administration of 120 mg/kg cimetidine depressed vitamin D 25-hydroxylase activity by 22%, and the same dose of isoniazid inhibited activity by 26%. The effect of long-term cimetidine therapy on vitamin D metabolism requires further evaluation.
Bergner P. Drug Herb Interactions. Medical Herbalism 1997;9(2):1.
Cupp MJ. Herbal remedies: adverse effects and drug interactions. Am Fam Physician 1999 Mar 1;59(5):1239-1245.
Force RW, Nahata MC. Effect of histamine H2-receptor antagonists on vitamin B12 absorption.
Ann Pharmacother 1992 Oct;26(10):1283-1286.
Abstract: OBJECTIVE: To discuss the potential of histamine H2-receptor antagonists (H2RAs) to cause malabsorption of vitamin B12 (cyanocobalamin). DATA SOURCES: Pertinent literature was identified via a MEDLINE search. Journals and references cited in published articles also were used as data sources. STUDY SELECTION: Studies evaluating the effect of H2RAs on vitamin B12 absorption were reviewed. DATA SYNTHESIS: H2RAs decrease acid secretion by the gastric parietal cells. Gastric acid and pepsin produced by these cells are required for the cleavage of vitamin B12 from dietary sources. Intrinsic factor (IF), also produced by gastric parietal cells, is required for vitamin B12 absorption from the gastrointestinal tract. Although H2RAs have not conclusively been shown to decrease IF secretion, studies have demonstrated a significant reduction in food-bound vitamin B12 absorption secondary to decreased acid secretion in patients taking these drugs. CONCLUSIONS: H2RAs have the potential to cause vitamin B12 deficiency. This may be important in patients with inadequate stores of vitamin B12 (e.g., poor diet), particularly those receiving H2RA therapy continuously for more than two years. Healthcare providers should be aware of this potential adverse effect.
Miller LG. Herbal medicinals: selected clinical considerations focusing on known or potential drug-herb interactions.
Arch Intern Med 1998 Nov 9;158(20):2200-2211.
Odes HS, Fraser GM, Krugliak P, Lamprecht SA, Shany S. Effect of cimetidine on hepatic vitamin D metabolism in humans.
Digestion 1990;46(2):61-64.
Abstract: Cimetidine inhibits the action of vitamin D-hydroxylase (a hepatic mixed-function oxidase) in the rat. Therefore, the hypothesis was tested that this H2 receptor antagonist would affect vitamin D metabolism in humans. Nine adult patients were treated with 400 mg cimetidine orally twice daily during a period from winter to summer, when days were becoming longer. Serum levels of 25-hydroxyvitamin D, 24,25-dihydroxyvitamin D and 1,25-dihydroxyvitamin D were monitored before treatment, after 4 weeks of treatment, and 1 month after cessation of treatment. No seasonal increase in the level of 25-hydroxyvitamin D was observed during the period of treatment, but the level rose significantly after withdrawal of the drug. The other hydroxylates of vitamin D were not affected. Levels of albumin, total calcium, phosphorus and alkaline phosphatase remained normal. The data suggest that short-term treatment with cimetidine could potentially perturb vitamin D metabolism in man.
Pourbaix S, Desager JP, Hulhoven R, Smith RB, Harvengt C. Pharmacokinetic
consequences of long term coadministration of cimetidine and triazolobenzodiazepines, alprazolam and triazolam, in healthy subjects.
Int J Clin Pharmacol Ther Toxicol 1985 Aug;23(8):447-451.
Russell RM, Golner BB, Krasinski SD, Sadowski JA, Suter PM, Braun CL Effect of antacid and H2 receptor antagonists on the intestinal absorption of folic acid. J Lab Clin Med 1988 Oct;112(4):458-463.
Abstract: Intestinal folic acid transport is a saturable process with a pH optimum of 5.5 to 6.0. Because of the possible effects of antacids and acid-lowering drugs on the pH of the proximal small intestine, the influence of these drugs on folic acid absorption was studied by using tritium-labeled pteroylmonoglutamic acid (PGA) in 30 subjects (21 women, nine men) of 56 to 89 years of age. Both cimetidine and an antacid containing aluminum and magnesium hydroxide reduced folate absorption from a liquid formula meal (p less than 0.01, p less than 0.001, respectively). Although ranitidine also caused a fall in folic acid absorption from the liquid meal, the change was not statistically significantly different from when PGA was given with the meal alone. Both histamine receptor antagonists tended to maintain a high intraluminal pH in the proximal small intestine after meals, which in part could explain the inhibition of folate absorption. However, neither drug was found to chemically interact with folic acid, and neither drug inhibited the dihydrofolate reductase. The antacid was found to precipitate folic acid at a pH of greater than 4.0, thus removing it from the aqueous phase. This appears to be the explanation for the lowered folate absorption in the presence of antacid. Although the effects of these drugs on reducing folic acid absorption were relatively small, such reductions could become clinically significant in chronic antacid or H2 receptor antagonist use or intensive antacid or H2 receptor antagonist use by individuals eating diets that are marginal in folate content.
Salom IL, Silvis SE, Doscherholmen A. Effect of cimetidine on the absorption of vitamin B12.
Scand J Gastroenterol 1982 Jan;17(1):129-131.
Abstract: The effect of cimetidine on the absorption of orally administered crystalline or food (egg yolk-bound) vitamin B12 (B12) was studied in 13 patients. Absorption or crystalline B12 was normal and not significantly changed by cimetidine. In contrast, the uptake of food-bound B12 decreased in all patients, from a mean of 5.3% without the drug to 2.5% after it, a fall of 53% (p less than 0.0001). This impairment of B12 absorption raises the possibility that long-term, full-dose therapy with cimetidine may produce B12 deficiency similar to that seen in other hypochlorhydric states. Our data indicate that cimetidine-induced B12 malabsorption would not be detected by the standard Schilling test.
Streeter AM, Goulston KJ, Bathur FA, Hilmer RS, Crane GG, Pheils MT. Cimetidine and malabsorption of
cobalamin. Dig Dis Sci 1982 Jan;27(1):13-16.
Vazquez Arnedo M, Pastor Fanco A, Bestue Fuster JM. [Cimetidine and toxic hepatitis].
Rev Esp Enferm Apar Dig. 1987 Jan;71(1):66-68. [Article in Spanish]