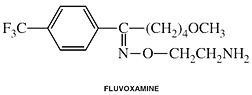

Fluvoxamine
Brand Names: LuvoxPlease read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
Cappiello A, McDougle CJ, Malison RT, Heninger GR, Price LH. Yohimbine augmentation of fluvoxamine in refractory depression: a single-blind study.
Biol Psychiatry 1995 Dec 1;38(11):765-767.
Chan BS, Graudins A, Whyte IM, Dawson AH, Braitberg G, Duggin GG. Serotonin syndrome resulting from drug interactions.
Med J Aust 1998 Nov 16;169(10):523-525.
Abstract: We describe six patients diagnosed with serotonin syndrome after exposure to drugs with serotonergic activity. Drug interactions occurred as a result of a combination of tricyclic antidepressants, selective serotonin reuptake inhibitors, selective noradrenaline reuptake inhibitors or monoamine oxidase inhibitors. Management included supportive care and the use of non-specific serotonin antagonists (cyproheptadine, benzodiazepines and chlorpromazine). All patients made uneventful recoveries.
Demott K. St. John’s wort tied to serotonin syndrome. Clin Psychiatr News 1998;26:28.
Steven Dentali, Ph.D., Dentali Associates: Natural Products Consulting Services, Troutdale, OR.
Gill M, LoVecchio F, Selden B. Serotonin syndrome in a child after a single dose of fluvoxamine. Ann Emerg Med 1999 Apr;33(4):457-459.
Abstract: Serotonin syndrome, a potentially fatal iatrogenic complication of psychopharmacologic therapy, is most commonly reported with combinations of serotonergic medications. Serotonin syndrome is characterized by alterations in cognition, behavior, autonomic, and central nervous system function as a result of increased postsynaptic serotonin receptor agonism. We present the first reported case of serotonin syndrome after a single dose of fluvoxamine in a pediatric patient after ingestion of a single supratherapeutic dose of fluvoxamine.
Goddard AW, Woods SW, Sholomskas DE, Goodman WK, Charney DS, Heninger GR. Effects of the serotonin reuptake inhibitor fluvoxamine on yohimbine-induced anxiety in panic disorder.
Psychiatry Res 1993 Aug;48(2):119-133.
Abstract: To assess the effects of the selective serotonin reuptake blocker fluvoxamine on noradrenergic function in patients with panic disorder, an intravenous yohimbine challenge test was administered to eight patients with panic disorder before and after 8 weeks of fluvoxamine treatment and to a parallel group of eight patients treated with placebo. Fluvoxamine treatment reduced yohimbine-induced anxiety while placebo treatment had no effect on this variable. Both fluvoxamine and placebo treatment had little effect on biochemical or physiologic responses to yohimbine.
Gordon JB. SSRIs and St. John’s wort: possible toxicity? Am Fam Phys 1998 Mar 1;57(5):950-953. (Letter)
Jeppesen U, Loft S, Poulsen HE, Brsen K. A fluvoxamine-caffeine interaction study.
Pharmacogenetics 1996 Jun;6(3):213-222.
Abstract: The selective serotonin reuptake inhibitor fluvoxamine is a very potent inhibitor of the liver enzyme CYP1A2, which is the major P450 catalysing the biotransformation of caffeine. Thus, a pharmacokinetic study was undertaken with the purpose of documenting a drug-drug interaction between fluvoxamine and caffeine. The study was carried out as a randomized, in vivo, cross-over study including eight healthy volunteers. In Period A of the study, each subject took 200 mg caffeine orally, and in Period B, the subjects took fluvoxamine 50 mg per day for 4 days and 100 mg per day for 8 days. On day 8 in Period B, the subjects again ingested 200 mg caffeine. After caffeine intake, blood and urine were sampled at regular intervals. Caffeine and its three primary demethylated metabolites, paraxanthine, theobromine and theophylline in plasma and the same four compounds plus 11 more metabolites in urine were assayed by HPLC. During fluvoxamine, the median of the total clearance of caffeine decreased from 107 ml min-1 to 21 ml min-1 and the half-life increased from 5 to 31 h. The N3-demethylation clearance of caffeine to paraxanthine decreased from 46 to 9 ml min-1; the N1- and N7-demethylation clearances decreased from 21 to 9 ml min-1 and from 14 to 6 ml min-1, respectively. The results confirm that CYP1A2 is the main enzyme catalysing the biotransformation of caffeine, in particular the N3-demethylation and partly the N1- and N7-demethylation. The results indicate that intake of caffeine during fluvoxamine treatment may lead to caffeine intoxication. Finally, our study provides additional evidence that fluvoxamine can be used to probe CYP1A2 in drug metabolism.
Rasmussen BB, Nielsen TL, Brosen K. Fluvoxamine is a potent inhibitor of the metabolism of caffeine in vitro.
Pharmacol Toxicol 1998 Dec;83(6):240-245.
Abstract: The selective serotonin re-uptake inhibitor, fluvoxamine, is a very potent inhibitor of CYP1A2, and accordingly causes pharmacokinetic interactions with drugs metabolised by CYP1A2, such as caffeine, theophylline, imipramine, tacrine and clozapine. Interaction between caffeine and fluvoxamine has been described in vivo, leading to lowering of total clearance of caffeine by 80% during fluvoxamine intake. The main purpose of the present study was to evaluate this interaction in vitro in human liver microsomes. A high-performance liquid chromatography method was developed in order to assay 1,3-dimethylxanthine, 1,7-dimethylxanthine, 3,7-dimethylxanthine and 1,3,7-trimethyluric acid formed from caffeine by human liver microsomes. The limit of detection was 0.06 nmol.mg protein-1.hr-1. As expected, fluvoxamine was a very potent inhibitor of the formation of the N-demethylated caffeine metabolites, displaying Ki values of 0.08-0.28 microM. The formation of 1,7-dimethylxanthine was virtually abolished by 10 microM of fluvoxamine, indicating that the N3-demethylation of caffeine is almost exclusively catalysed by CYP1A2. The CYP3A4 inhibitors, ketoconazole and bromocriptine, inhibited 1,3,7-trimethyluric acid formation with Kis of 0.75 microM and 5 microM, respectively, thus further supporting the involvement of CYP3A4 in the 8-hydroxylation of caffeine. The study shows that fluvoxamine, as expected, is a potent inhibitor of the metabolism of caffeine in vitro.
Spigset O, Carleborg L, Hedenmalm K, Dahlqvist R. Effect of cigarette smoking on fluvoxamine pharmacokinetics in humans.
Clin Pharmacol Ther 1995 Oct;58(4):399-403.
Abstract: OBJECTIVES: Although fluvoxamine inhibits the biotransformation of drugs known to be metabolized by CYP1A2, there are no data available with regard to the importance of CYP1A2 for the metabolism of fluvoxamine itself. Because smoking induces the metabolism of drugs catalyzed by CYP1A2, this study investigated the pharmacokinetics of fluvoxamine in smokers and nonsmokers. METHODS: The serum concentration of fluvoxamine was determined by high-performance liquid chromatography for 48 hours after oral administration of a single dose of 50 mg fluvoxamine to 12 smokers (> or = 10 cigarettes per day) and 12 nonsmokers. RESULTS: The smokers had significantly lower areas under the serum concentration-time curve and significantly lower maximal serum concentrations than the nonsmokers (mean +/- SD, 771 +/- 346 versus 1110 +/- 511 nmol.hr.L-1 [p = 0.012] and 39.1 +/- 17.3 versus 57.7 +/- 21.5 nmol.L-1 [p = 0.012], respectively). The terminal elimination half-life did not differ significantly between smokers and nonsmokers (10.1 +/- 1.9 and 10.7 +/- 2.3 hours, respectively). The oral clearance was high among both smokers (4.1 +/- 1.9 L.min-1) and nonsmokers (3.3 +/- 2.7 L.min-1; difference not significant). CONCLUSION: Smokers had lower serum concentrations of fluvoxamine than nonsmokers after a single oral dose of fluvoxamine. This finding is consistent with a possible role of CYP1A2 in fluvoxamine metabolism.