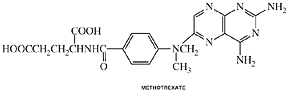
Methotrexate
Brand Names: Amethopterin, Folex, Methotrate, Mexate, Rheumatrex
Clinical Names: Methotrexate
Summary
generic name: Methotrexate
trade names: Amethopterin®, Folex®, Methotrate®, Mexate®, Rheumatrex®
type of drug: Cytotoxic chemotherapy.
mechanism: Methotrexate works by interfering with the activation of folate, specifically by inhibiting dihydrofolatereductase, thereby disrupting DNA replication for all cells, but especially rapidly growing cancer cells.
used to treat: Cancer, psoriasis and rheumatoid arthritis.
overview of interactions:
• nutrient affected by drug: Folic Acid
• nutrient interactions affecting drug performance and toxicity: Folic Acid
• nutrient affecting drug performance: Vitamin A
• nutrient affecting drug performance: Vitamin B12
(Cobalamin)
Interactions
nutrient affected by drug: Folic Acid
• mechanism: The primary mechanism of methotrexate relies upon interfering with the activation of folic acid and the degree of folate depletion during methotrexate therapy depends primarily upon the weekly administered dose.
nutrient interactions affecting drug performance and toxicity: Folic Acid
Methotrexate has varying patterns of use for different conditions and the relationship of folic acid to the drug mechanism changes accordingly. This difference is especially important in individuals using methotrexate as a chemotherapeutic agent and those taking it for rheumatoid arthritis.
• nutritional concerns with chemotherapy: Since methotrexate's interference with folic acid metabolism is intentional, individuals prescribed this drug for cancer treatment should limit their supplementation of folic acid to a maximum of 400 mcg per day. Consultation with your prescribing physician or other qualified healthcare provider is important because use of folic acid at higher levels might work contrary to the drug's therapeutic intention. However, this caution against folate supplementation does not extend to individuals taking chemotherapeutic agents other than methotrexate.
• research: As the use of methotrexate for the treatment of rheumatoid arthritis has evolved so has the understanding of the use of folic acid by individuals undergoing therapy. While use of methotrexate for treatment of rheumatoid arthritis has grown in recent years, over 30% of patients abandon treatment because of drug-related side effects. Initially researchers assumed that methotrexate's effects on folic acid were the source of its presumed benefits in cases of rheumatoid arthritis, as in chemotherapeutic uses. However, with time and further research, practice has shifted to support the supplemental use of folic acid to counter the adverse effects of methotrexate in these cases for several reasons. First, its well-proven ability to reduce toxic effects of methotrexate. Second, methotrexate causes folate deficiency and the folate nutriture of patients taking even low dose methotrexate declines precipitously without folic acid supplementation. Third, plasma homocysteine levels can increase significantly in those taking methotrexate but not folate; thereby significantly increasing risk of cardiovascular disease. These and other benefits are gained with no apparent loss of antirheumatic effect. Most researchers have found that folic acid levels were not related to parameters of disease activity and concluded that methotrexate does not exert its action in RA primarily by inhibiting dihydrofolatereductase.
(Alarcon GS, Morgan SL. Arthritis Rheum 1997 Feb;40(2):391; van Ede AE, et al.
Semin Arthritis Rheum 1998 Apr;27(5):277-292; Hunt PG, et al. J Rheumatol
1997 Nov;24(11):2230-2232; Leeb BF, et al. Clin Exp Rheumatol 1995 Jul-Aug;13(4):459-463; Morgan SL, et al.
J Rheumatol 1998 Mar;25(3):441-446; Shiroky JB. Rheum Dis Clin North Am 1997 Nov;23(4):969-680.)
• nutritional support with rheumatoid conditions: Though its use is not universally accepted, the ability of folic acid (or folinic acid) to reduce methotrexate toxicity in individuals being treated for rheumatoid arthritis has made its use an important adjunct in enabling patients to tolerate methotrexate. At this time, research supports several rationales for substantial folate supplementation by individuals taking methotrexate for rheumatoid arthritis. Beyond the prevention of methotrexate toxicity, the prevention or treatment of folate deficiency and the prevention of hyperhomocysteinemia further contribute to the therapeutic value of supplementation with high doses of folic acid. According to the several studies cited, a daily dose of 1000-5000 mcg of folic acid or 2.5-5 mg of folinic acid (an activated form of folic acid) can substantially reduce the adverse effects of methotrexate without compromising its therapeutic effect in rheumatoid patients.
(Morgan SL, et al. J Rheumatol 1998 Mar;25(3):441-446; Shiroky JB. Rheum Dis Clin North Am
1997 Nov;23(4):969-980; Kamen B. Semin Oncol 1997 Oct;24(5 Suppl 18):S18-30-S18-39; Morgan, SL, et al.
Ann Intern Med 1994;121:833-841; Ortiz Z, et al. J Rheumatol 1998 Jan;25(1):36-43; Shiroky JB, et al.
Arthrit Rheum 1993;36:795.)
nutrient affecting drug performance: Vitamin A
• mechanism: Researchers have found that vitamin A enhances antitumor activity in animals taking methotrexate.
(Nakagawa, M, et al. Jpn J Cancer Res 1985;76:887-894.)
• nutritional support: Individuals using methotrexate for the treatment of tumors should consult their prescribing physician and/or a nutritionally-oriented healthcare professional before initiating or increasing supplementation of vitamin A.
nutrient affecting drug performance: Vitamin B12
(Cobalamin)
• mechanism: Given methotrexate's intentional interference with normal functioning of folic acid metabolism, problems with vitamin B12 deficiency can be expected. Further, vitamin B12 and folic acid work together to control homocysteine levels. Investigators have found that patients taking methotrexate have lower B12/RBC levels than in controls, but that serum levels of B12 were not different. They concluded that folate depletion may be related to B12 deficiency in red blood cells.
(Leeb BF, et als. Clin Exp Rheumatol 1995 Jul-Aug;13(4):459-463.)
• nutritional support: In no circumstances is methotrexate-induced Vitamin B12 deficiency considered important to the drug's therapeutic efficacy. However, since folate deficiency inherently results from methotrexate use, some support to counterbalance the unintentional adverse effects on Vitamin B12 levels would appear valuable. While supplementation with vitamin B12 is harmless and indicated, individuals receiving treatment with methotrexate should consult their prescribing physician and/or a nutritionally-oriented healthcare professional before initiating or increasing supplementation of Vitamin B12. In such cases, supplementation is often up to or greater than 1,000 mcg of vitamin B12 per day or be provided by a physician via vitamin B12 injections.


Please read the disclaimer concerning the intent
and limitations of the information provided here.
Do not rely solely on the information in this article.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
References
Alarcon GS, Morgan SL. Guidelines for folate supplementation in rheumatoid arthritis patients treated with methotrexate: comment on the guidelines for monitoring drug therapy.
Arthritis Rheum 1997 Feb;40(2):391; discussion 391-392. (Letter)
Duell PB, Malinow MR. Homocyst(e)ine: an important risk factor for atherosclerotic vascular disease.
Curr Opin Lipidol 1997 Feb;8(1):28-34. (Review)
Abstract: Homocysteine is an intermediate compound formed duringmetabolism of methionine. The results of many recent studies have indicated that elevated plasma levels of homocyst(e)ine are associated with increased risk of coronary atherosclerosis, cerebrovascular disease, peripheral vascular disease, and thrombosis. The plasma level of homocyst(e)ine is dependent on genetically regulated levels of essential enzymes and the intake of folic acid, vitamin B6 (pyridoxine), and vitamin B12 (cobalamin). Impaired renal function, increased age, and pharmacologic agents (e.g. nitrous oxide, methotrexate) can contribute to increased levels of homocyst(e)ine. Plausible mechanisms by which homocyst(e)ine might contribute to atherogenesis include promotion of platelet activation and enhanced coagulability, increased smooth muscle cell proliferation, cytotoxicity, induction of endothelial dysfunction, and stimulation of LDL oxidation. Levels of homocysteine can be reduced with pharmacologic doses of folic acid, pyridoxine, vitamin B12, or betaine, but further research is required to determine the efficacy of this intervention in reducing morbidity and mortality associated with atherosclerotic vascular disease.
Fiskerstrand T, Ueland PM, Refsum H. Folate depletion induced by methotrexate affects methionine synthase activity and its susceptibility to inactivation by nitrous oxide.
J Pharmacol Exp Ther 1997 Sep;282(3):1305-1311.
Abstract: We compared the effects of methotrexate (MTX) and nitrous oxide on the methionine (Met) synthase system in two variants of a human glioma cell line. The cells were protected from cytotoxic effect of MTX by adding thymidine and hypoxanthine to the cell culture medium. MTX (0-1 microM) was associated with a dose- and time-dependent reduction in 5-methyltetrahydrofolate (5-methyl-THF) in both cell lines. Already after 3 hr of exposure, 5-methyl-THF was reduced by 50% and after additional 48 hr, the level was undetectable. In addition to reduction in folate level, homocysteine (Hcy) remethylation in intact cells was markedly inhibited as judged by an increased export of Hcy from the cells, and Met synthase activity in cell extracts and level of cellular methylcobalamin (CH3Cbl) declined. MTX reduced Hcy remethylation and CH3Cbl level more efficiently than nitrous oxide. In both cell variants, the inactivation of Met synthase by nitrous oxide was almost completely prevented in cells pre-exposed to MTX. This indicates that there is no catalytic turnover in cells exposed to MTX, and emphasizes the importance of the sequence of administration for synergistic effect of this drug combination. In conclusion, our data show that MTX through depletion of 5-methyl-THF reduces both the Met synthase activity and the cellular CH3Cbl level. Moreover, the effect of MTX on the Hcy remethylation is more pronounced than the inhibition caused by nitrous oxide. These observations should be taken into account in studies on MTX pharmacodynamics.
Hunt PG, Rose CD, McIlvain-Simpson G, Tejani S. The effects of daily intake of folic acid on the efficacy of methotrexate therapy in children with juvenile rheumatoid arthritis. A controlled study.
J Rheumatol 1997 Nov;24(11):2230-2232.
Abstract: OBJECTIVE: To determine the effect of 1 mg/day of folic acid on the efficacy of methotrexate (MTX) to control disease activity in children with juvenile rheumatoid arthritis (JRA). METHODS: Randomized, double blind, placebo controlled, crossover trial of 13 weeks' duration. Nineteen children with the diagnosis of JRA, fulfilling the American College of Rheumatology diagnostic criteria, who had been receiving MTX for at least 6 months and whose disease status had remained stable for at least one month before entry were enrolled in the study. Subjects were randomly assigned to receive 1 mg/day of liquid folic acid or a liquid placebo for 6 weeks, followed by a one week washout period, and subsequent crossover to the alternate form for another 6 weeks. Disease activity indicators, including swollen joint count, duration of morning stiffness, physician and patient global assessment, and C-reactive protein, were assessed at study entry and at 6 and 13 weeks. RESULTS: One patient flared during the first 2 weeks while taking placebo, requiring study withdrawal and exclusion from outcome analysis. For the remaining 18 patients, there was no statistical difference in disease activity indicators with folic acid treatment compared to placebo. CONCLUSION: Supplementation with 1 mg/day of folic acid may not affect the clinical efficacy of oral weekly MTX in children with JRA.
Kamen B. Folate and antifolate pharmacology. Semin Oncol 1997 Oct;24(5 Suppl 18):S18-30-S18-39. (Review)
Abstract: Folic acid is a water-soluble vitamin associated with the other B vitamins. In its fully reduced form (tetrahydrofolate), folate serves as a 1-carbon donor for synthesis of purines and thymidine as well as in the remethylation cycle of homocysteine to methionine. Folate is essential for normal cell growth and replication. It therefore is not surprising that folate analogues have served and continue to serve well as antibiotics and cytotoxic drugs in the treatment of cancer, autoimmune diseases, psoriasis, and bacterial and protozoal infections. During the past 50 years, many of the enzymes requiring folate as a co-factor (ie, thymidylate synthase), and molecules critical in folate homeostasis (ie, the reduced folate carrier, folylpolyglutamate synthase), have been purified and even crystallized. The genes have been cloned, sequenced, and mapped, providing detailed knowledge of their regulation and three-dimensional structure. This has, in part, led to the rational synthesis of a large number of folate analogues that differ from methotrexate, the "classical antifolate," in transport, metabolism, and intracellular targets. Currently, several new folate analogues with unique biochemical properties and clinical applications are being tested. The goals of this brief review are to review folate homeostasis, to highlight the similarities and differences between natural folate and antifolates with respect to biochemistry and metabolism, and to present the pharmacology of methotrexate and several next-generation folate analogues, such as trimetrexate and raltritrexed, with an emphasis on mechanisms of drug resistance.
Leeb BF, Witzmann G, Ogris E, Studnicka-Benke A, Andel I, Schweitzer H, Smolen JS. Folic acid and cyanocobalamin levels in serum and erythrocytes during low-dose methotrexate therapy of rheumatoid arthritis and psoriatic arthritis patients.
Clin Exp Rheumatol 1995 Jul-Aug;13(4):459-463.
Abstract: OBJECTIVE. To compare folic acid (FA) levels in patients being treated with methotrexate (MTX) with those of untreated patients in order to investigate potential folate depletion by MTX and its possible relationship to the drug's efficacy. METHODS. In 33 patients on low-dose MTX therapy and in 24 controls, FA and cyanocobalamin (B12) levels were determined in serum and red blood cells (RBC). In addition, MTX levels in the RBC and serum were measured, and clinical and laboratory measures of disease activity were evaluated. RESULTS. MTX treated patients had lower FA levels than controls (median 4.36 vs 7.37 ng/ml, p < 0.001). A significant correlation between serum FA and MTX/RBC (p < 0.01) and between the weekly dose and MTX/RBC (p < 0.01) was seen. There was apparently no correlation between FA and the cumulative total MTX. MTX patients had lower B12/RBC levels than the controls (p < 0.001); the serum levels of B12 were not different. Clinical features, ESR and CRP did not correlate with FA, B12 or MTX levels. CONCLUSIONS. The degree of folate depletion during MTX therapy depends primarily upon the weekly administered dose. Folate depletion may be related to B12 deficiency in RBC. Since FA levels were not related to parameters of disease activity it is conceivable that MTX does not exert its action in RA primarily by inhibiting dihydrofolatereductase. Therefore, additional folate compounds, if necessary, should not lead to a reduction in the efficacy of MTX.
Morgan SL, Baggott JE, Lee JY, Alarcon GS. Folic acid supplementation prevents deficient blood folate levels and hyperhomocysteinemia during longterm, low dose methotrexate therapy for rheumatoid arthritis: implications for cardiovascular disease prevention.
J Rheumatol 1998 Mar;25(3):441-446. Abstract: OBJECTIVE: To determine the effect of longterm methotrexate (MTX) therapy and folic acid supplementation on folate nutriture and homocysteine levels in patients with rheumatoid arthritis. METHODS: A double blind, placebo controlled trial lasting one year was conducted at one academic medical center. A total of 79 patients taking low dose MTX were followed up to one year. The patients were randomized to receive placebo or 5 or 27.5 mg folic acid supplementation per week. RESULTS: Plasma and erythrocyte folate levels and plasma homocysteine levels were determined. The folate nutriture of patients taking low dose MTX declined without folic acid supplementation. Plasma homocysteine levels increased significantly over a one year period in the placebo group. Low folate nutriture and hyperhomocysteinemia occurred with greater frequency in the placebo group than in the folic acid supplemented groups. CONCLUSION: For longterm, low dose MTX therapy, there are now at least 3 reasons to consider supplementation with folic acid (a low cost prescription): (1) to prevent MTX toxicity, (2) to prevent or treat folate deficiency, and (3) to prevent hyperhomocysteinemia, considered by many investigators to be a risk factor for cardiovascular disease.
Morgan, SL, Baggott, JE, Vaughn, WH, et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis.
Ann Intern Med 1994;121:833-841.
Nakagawa M, Yamaguchi T, Ueda H, Shiraishi N, Komiyama S, Akiyama S, Ogata J, Kuwano M. Potentiation by vitamin A of the action of anticancer agents against murine tumors.
Jpn J Cancer Res (Gann) 1985;76:887-894.
Abstract: Combinations of retinol palmitate (RP) and six different anticancer agents were examined to determine their effects on the life-span of mice bearing ascites sarcoma 180 or P388 leukemia. With ascites sarcoma 180, administration of a fixed dose of RP (3.3 mg/kg) considerably enhanced the antitumor effects of 5-fluorouracil (5-FU) (5 mg/kg, or 20 mg/kg), methotrexate (MTX) (0.5 mg/kg, or 1 mg/kg) and 1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea (ACNU) (12.5 mg/kg), when given by intraperitoneal injection. However RP failed to potentiate the antitumor effects of adriamycin (ADM) and 6-mercaptopurine (6-MP) against sarcoma 180. With P388 leukemia, RP (167 mg/kg, or 333 mg/kg) enhanced the antitumor effects of 6-MP (25 mg/kg, or 50 mg/kg), MTX (1 mg/kg, or 2 mg/kg), ADM (0.2 mg/kg), ACNU (5 mg/kg) and cis-dichlorodiammine-platinum (CDDP) (1 mg/kg) to a considerable extent, but it did not potentiate the antitumor effect of 5-FU. The combination of RP with ACNU or CDDP was particularly effective against P388 leukemia.
Ortiz Z, Shea B, Suarez-Almazor ME, Moher D, Wells GA, Tugwell P. The efficacy of folic acid and folinic acid in reducing methotrexate gastrointestinal toxicity in rheumatoid arthritis. A metaanalysis of randomized controlled trials.
J Rheumatol 1998 Jan;25(1):36-43.
Abstract: OBJECTIVE: To assess the efficacy of folic acid and folinic acid in reducing the mucosal and gastrointestinal (GI) side effects of low dose methotrexate (MTX) in patients with rheumatoid arthritis (RA). METHODS: A systematic review was carried out using the methods recommended by the Cochrane Collaboration. We used MEDLINE and performed hand searches that included bibliographic references, Current Contents, abstracts of rheumatology meetings, and 4 rheumatology journals to select double blind randomized controlled trials (RCT) in which adult patients with RA were treated with low doses of MTX (< 20 mg/week), concurrently with folic or folinic acid. The quality of the RCT was assessed. The overall treatment effect across trials (reduction in toxicity) was estimated using a fixed effects model. Disease activity was evaluated using standardized mean differences to ensure comparability across outcome measures. Sensitivity analyses were conducted evaluating different doses and the quality of the trials. Costs per month in different countries were compared. RESULTS: Of 11 trials retrieved, 7 met inclusion criteria. The total sample included 307 patients, of which 147 were treated with folate supplementation, 67 patients with folic, and 80 with folinic acid. A 79% reduction in mucosal and GI side effects was observed for folic acid [OR = 0.21 (95% CI 0.10 to 0.44)]. For folinic acid, a clinically but nonstatistically significant reduction of 42% was found [OR = 0.58 (95% CI 0.29 to 1.16)]. No major differences were observed between low and high doses of folic or folinic acid. Hematologic side effects could not be analyzed, since details by patients of each event were not provided. No consistent differences in disease activity variables were observed when comparing placebo and folic acid or folinic acid at low doses; patients receiving high dose folinic acid had increased tender and swollen joint counts. Substantial differences in costs across countries were found; folinic acid was more expensive. CONCLUSION: Our results support the protective effect of folate supplementation in reducing MTX side effects related to the oral and GI systems.
Shiroky JB. Folic acid and methotrexate in rheumatoid arthritis. Ann Intern Med
1996 Jan 1;124(1 Pt 1):73-74. (Letter)
Shiroky JB, Neville C, Esdaile JM, Choquette D, Zummer M, Hazeltine M, Bykerk V, Kanji M, St-Pierre A, Robidoux L, et al. Low-dose methotrexate with leucovorin (folinic acid) in the management of rheumatoid arthritis.
Arthrit Rheum 1993;36:795.
Abstract: OBJECTIVE. To determine whether the side effects of methotrexate can be decreased by the concurrent use of leucovorin, without affecting the efficacy of the methotrexate. METHODS. We conducted a multicenter randomized, double-blind, placebo-controlled trial of leucovorin administration, 2.5-5.0 mg orally, to be given 24 hours after the single, weekly, oral dose of methotrexate. Every 3 weeks for 52 weeks, patients were evaluated for rheumatic disease activity and side effects. Dosage adjustments for both methotrexate and leucovorin were made as needed, according to a defined protocol. The primary outcome evaluated was the frequency of study withdrawals because of side effects and/or inefficacy. Secondary outcomes evaluated included the frequency of side effects and the relative efficacy of methotrexate in the leucovorin and placebo treatment groups. RESULTS. Ninety-two evaluable patients were analyzed (44 took leucovorin and 48 placebo). Twenty-two patients withdrew early because of side effects unresponsive to our protocol, and 1 because of inefficacy; 17 had been taking placebo and 6 had been taking leucovorin (35% versus 14%, P < 0.02). The number of visits during which side effects were reported was reduced by almost 50% in the leucovorin treatment group (P < 0.001). There were significant reductions in the frequencies of all common side effects. At 52 weeks, disease activity was similar in both patient groups. CONCLUSION. The methotrexate-leucovorin protocol used significantly reduces common side effects of methotrexate therapy without significantly altering efficacy.
Shiroky JB. The use of folates concomitantly with low-dose pulse methotrexate.
Rheum Dis Clin North Am 1997 Nov;23(4):969-680. (Review)
Abstract: Toxicities related to low-dose weekly methotrexate are largely due to its antifolate properties. Preexisting folate deficiency is associated with methotrexate toxicity in some patients. At the onset of methotrexate therapy and throughout therapy, the physician should be vigilant regarding one or more nutrient deficiencies. A multivitamin and, where appropriate, specific daily folic acid supplements should be employed. The only regimen known presently (through controlled trials) to treat side effects is the low-dose folinic acid (leucovorin) protocol outlined herein. Folic acid may be helpful to treat mild gastrointestinal symptoms. Folinic acid supplementation should be considered prophylactically in those requiring methotrexate who are at increased risk of hepatic disease. Other possible factors besides methotrexate should always be considered with the onset of new patient complaints or laboratory abnormalities. Claims that folic acid therapy is safer and more convenient than folinic acid seem unwarranted when one reviews the literature carefully. Cost differences between folic acid supplementation and folinic acid supplementation have been exaggerated.
van Ede AE, Laan RF, Blom HJ, De Abreu RA, van de Putte LB. Methotrexate in rheumatoid arthritis: an update with focus on mechanisms involved in toxicity. Semin Arthritis Rheum
1998 Apr;27(5):277-292. (Review)
Abstract: OBJECTIVES: To provide an update of the current knowledge of the mechanism of action of low-dose methotrexate (MTX) in the treatment of patients with rheumatoid arthritis (RA), with an emphasis on the mechanisms involved in toxicity. We also considered strategies currently used to prevent or decrease toxicity of MTX. METHODS: We reviewed the literature dealing with the subjects of MTX treatment of RA, the mechanisms of action of low-dose MTX regarding efficacy and toxicity, and strategies used to prevent or decrease MTX toxicity. RESULTS: MTX is a fast working and effective second-line antirheumatic agent (SLA). Its use is limited mainly because of side effects. The mechanisms of action regarding efficacy and toxicity are probably determined by different metabolic pathways. Recent data indicate that the antiinflammatory effect of MTX is mediated by adenosine. However, MTX side effects can only partly be explained by folate antagonism and may also depend on its action on other related metabolic pathways. The latter include the homocysteine-methionine-polyamine pathway and purine metabolism. Variants in these metabolic routes (ie, the C677T mutation in the methylene-tetrahydrofolate reductase [MTHFR] gene), may predispose to the development of side effects. Currently the most promising strategy to decrease or prevent toxicity of MTX is concomitant prescription of folic acid or folinic acid. Other strategies are currently under investigation. CONCLUSIONS: MTX benefits a majority of RA patients. Approximately 30% of patients, however, abandon treatment because of drug-related side effects. Folic acid or folinic acid likely reduces MTX toxicity. More data, however, are needed to evaluate a potential detrimental effect on the antirheumatic efficacy of
MTX.

