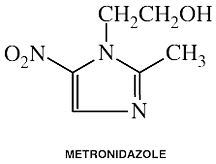

Metronidazole
Brand Names: FlagylPlease read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
Cina SJ, Russell RA, Conradi SE. Sudden death due to metronidazole/ethanol interaction.
Am J Forensic Med Pathol 1996 Dec;17(4):343-346.
De Martiis M, Fontana M, Assogna G, D'Ottavi R, D'Ottavi O. [Milk thistle (Silybum marianum) derivatives in the therapy of chronic hepatopathies].
Clin Ter 1980 Aug 15;94(3):283-315. [Article in Italian]
Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease.
Am J Gastroenterol 1998 Feb;93(2):139-143.
Abstract: Silymarin, derived from the milk thistle plant, Silybum marianum, has been used for centuries as a natural remedy for diseases of the liver and biliary tract. As interest in alternative therapy has emerged in the United States, gastroenterologists have encountered increasing numbers of patients taking silymarin with little understanding of its purported properties. Silymarin and its active constituent, silybin, have been reported to work as antioxidants scavenging free radicals and inhibiting lipid peroxidation. Studies also suggest that they protect against genomic injury, increase hepatocyte protein synthesis, decrease the activity of tumor promoters, stabilize mast cells, chelate iron, and slow calcium metabolism. In this article we review silymarin's history, pharmacology, and properties, and the clinical trials pertaining to patients with acute and chronic liver disease.
Harries DP, Teale KF, Sunderland G. Metronidazole and alcohol: potential problems.
Scott Med J. 1990 Dec;35(6):179-180.
Hrelia P, Murelli L, Paolini M, Cantelli-Forti G. In vivo protective role of antioxidants against genotoxicity of metronidazole and
azanidazole. Drugs Exp Clin Res 1987;13(9):577-583.
Abstract: The mutagenicity of metronidazole and azanidazole has been extensively reported. Previous experiments demonstrated, by means of the intrasanguineous host-mediated assay, that they significantly induced mutagenicity in liver, kidney and lung of mice. The treatment of mice with butylated hydroxyanisole (BHA) or butylated hydroxytoluene (BHT) by two different routes of administration (i.p. injection and oral intubation) significantly reduced liver- and kidney-mediated mutagenicity of azanidazole and metronidazole. No significant differences were observed between the routes of treatment in terms of protective effect on genotoxicity of azanidazole in the considered organs, whereas i.p. administration was the most suppressive on the mutagenicity of metronidazole. Even if BHT was the most effective agent in preventing mutation induction in mice, a detectable toxicity, in terms of increased mutagenicity, was evaluated in the liver. Evidence of lung abnormalities was also seen. The results suggest that the possible adverse effects on biological systems limit the prophylactic use of BHA and BHT in preventing the action of chemical carcinogens in man.
Morazzoni P, Bombardelli E. Silybum marianum (Carduus marianus). Fitoterapia 1995;66:3-42 (Review).
Muzes G, Deak G, Lang I, Nekam K, Niederland V, Feher J. [Effect of silimarin (Legalon) therapy on the antioxidant defense mechanism and lipid peroxidation in alcoholic liver disease].
Orv Hetil 1990 Apr 22;131(16):863-866. [Article in Hungarian]
Abstract: A double blind study. Antioxidant and antiperoxidative effects of the free radical scavenger agent silymarin (Legalon) were investigated in patients with chronic alcoholic liver disease in a double blind clinical trial. Six month treatment (at a daily dose of 420 mg) with silymarin significantly enhanced the originally low superoxide dismutase activity of erythrocytes and lymphocytes and also restored the diminished superoxide dismutase expression on lymphocytes as measured by flow-cytofluorimetry. In addition, silymarin therapy markedly increased the serum level of ree--SH groups and the activity of glutathione peroxidase. In contrast, a considerable fall in serum malondialdehyde concentration was detected in patients having received silymarin. However, in case of placebo-treated patients the above mentioned parameters of antioxidant defense system and lipid peroxidation failed to change significantly. These data indirectly suggest that antioxidant, antiperoxidative effects might be important factors in the mechanism of hepatoprotective action of silymarin.
Plosker GL. Possible interaction between ethanol and vaginally administered metronidazole.
Clin Pharm. 1987 Mar;6(3):189, 192-193.
Threlkeld DS, ed. Systemic Anti-Infectives, Metronidazole. In: Facts and Comparisons Drug Information. St. Louis, MO: Facts and Comparisons, Nov 1992.
Tunguy-Desmarais GP. Interaction between alcohol and metronidazole. S Afr Med J. 1983 May 28;63(22):836.