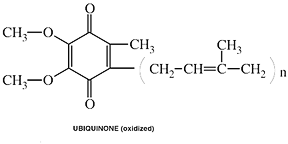

Coenzyme Q10
Common Names: CoQ10, Ubiquinone, UbidecarenonePlease read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
Baum H. New Scientist May 24, 1991, 24.
Beyer RE. An analysis of the role of coenzyme Q in free radical generation, and as an antioxidant.
Biochem Cell Biol 1992 70(6):390-403. (Review)
Abstract: The vital role of coenzyme Q in mitochondrial electron transfer and its regulation, and in energy conservation, is well established. However, the role of coenzyme Q in free oxyradical formation and as an antioxidant remains controversial. Demonstration of the existence of the semiquinone form of coenzyme Q during electron transport, coupled with recent evidence that hydrogen peroxide (but not molecular oxygen) may act as an oxidant of the semiquinone, suggests that the highly reactive OH. radical may be formed from the semiquinone. On the other hand, data exist implicating the Fe-S species as the source of electron transfer chain, free radical production. Additional data exist suggesting instead that the unpaired electron of the coenzyme Q semiquinone most likely dismutases superoxide radicals. These concepts and those arising from observations at several levels of organization including subcellular systems, intact animals, and human subjects in the clinical setting, supporting the concept of reduced coenzyme Q as an antioxidant, will be presented. The results of recent studies on the interaction between the two-electron quinone reductase--DT diaphorase and coenzyme Q10 will be presented. The possibility that superoxide dismutase may interact with reduced coenzyme Q, in conjunction with DT diaphorase inhibiting its autoxidation, will be described. The regulation of cellular coenzyme Q concentrations during oxidative stress accompanying aerobic exercise, resulting in increased protection from free radical damage, will also be presented.
Beyer RE. The role of ascorbate in antioxidant protection of biomembranes: interaction with vitamin E and coenzyme Q.
J Bioenerg Biomembr. 1994 Aug;26(4):349-358. (Review)
Abstract: One of the vital roles of ascorbic acid (vitamin C) is to act as an antioxidant to protect cellular components from free radical damage. Ascorbic acid has been shown to scavenge free radicals directly in the aqueous phases of cells and the circulatory system. Ascorbic acid has also been proven to protect membrane and other hydrophobic compartments from such damage by regenerating the antioxidant form of vitamin E. In addition, reduced coenzyme Q, also a resident of hydrophobic compartments, interacts with vitamin E to regenerate its antioxidant form. The mechanism of vitamin C antioxidant function, the myriad of pathologies resulting from its clinical deficiency, and the many health benefits it provides, are reviewed.
Combs AB, Porter TH, Folkers K. Anticoagulant activity of a naphthoquinone analog of vitamin K and an inhibitor of coenzyme Q10-enzyme systems.
Res Commun Chem Pathol Pharmacol. 1976 Jan;13(1):109-114.
Abstract: Synthetic 2-hydroxy-3-h-dodecylmercapto-1,4-naphthoquinone is an analog of both vitamin K1 and coenzyme Q10. This naphthoquinone analog is an effective inhibitor of coenzyme Q10-enzymes of mammalian mitochondria, which are components of electron transfer mechanisms of respiration and coupled oxidative phosphorylation. This analog increased the prothrombin time in rats when it was administered orally or parenterally. Vitamin K1 reversed the prothrombin time increase, but that form of coenzyme Q, hexahydrocoenzyme Q4, which has the same phytyl side chain as vitamin K1, did not reverse the increase, constituting the biological differentiation between vitamin K and coenzyem Q. Two benzoquinone analogs of coenzyme Q10, 5-n-octadecylmercapto-2,3-dimethoxy-1,4-benzoquinone and 5-beta-naphthylmercapto-2,3-dimethoxy-1,4-benzoquinone, the latter being a strong inhibitor of coenzyme Q10-enzymes, did not increase the prothrombin time under comparable conditions.
Donchenko HV, Chahovets' RV. Changes in ubiquinone content of the liver caused by cortisone acetate in normal and vitamin A-deficient rats.
Fed Proc Transl Suppl 1965 Nov-Dec;24(6):983-985.
Folkers K. Basic chemical research on coenzyme Q10 and integrated clinical research on therapy of diseases. In G. Lenaz, Ed.
Coenzyme Q. New York: John Wiley and Sons, 1985.
Folkers K, Langsjoen P, Eds. In: Folkers K, Littarru GP, Yamagami T, Eds. Biochemical and Clinical Aspects of Coenzyme Q, Volume 6. Amsterdam, Elsevier Science Publ, 1991: 449-452.
Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, Tamagawa H. Lovastatin decreases coenzyme Q levels in humans.
Proc Natl Acad Sci U S A. 1990 Nov;87(22):8931-8934.
Abstract: Lovastatin is clinically used to treat patients with hypercholesterolemia and successfully lowers cholesterol levels. The mechanism of action of lovastatin is inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, an enzyme involved in the biosynthesis of cholesterol from acetyl-CoA. Inhibition of this enzyme could also inhibit the intrinsic biosynthesis of coenzyme Q10 (CoQ10), but there have not been definitive data on whether lovastatin reduces levels of CoQ10 as it does cholesterol. The clinical use of lovastatin is to reduce a risk of cardiac disease, and if lovastatin were to reduce levels of CoQ10, this reduction would constitute a new risk of cardiac disease, since it is established that CoQ10 is indispensable for cardiac function. We have conducted three related protocols to determine whether lovastatin does indeed inhibit the biosynthesis of CoQ10. One protocol was done on rats, and is reported in the preceding paper [Willis, R. A., Folkers, K., Tucker, J. L., Ye, C.-Q., Xia, L.-J. & Tamagawa, H. (1990) Proc. Natl. Acad. Sci. USA 87, 8928-8930]. The other two protocols are reported here. One involved patients in a hospital, and the other involved a volunteer who permitted extraordinary monitoring of CoQ10 and cholesterol levels and cardiac function. All data from the three protocols revealed that lovastatin does indeed lower levels of CoQ10. The five hospitalized patients, 43-72 years old, revealed increased cardiac disease from lovastatin, which was life-threatening for patients having class IV cardiomyopathy before lovastatin or after taking lovastatin. Oral administration of CoQ10 increased blood levels of CoQ10 and was generally accompanied by an improvement in cardiac function. Although a successful drug, lovastatin does have side effects, particularly including liver dysfunction, which presumably can be caused by the lovastatin-induced deficiency of CoQ10.
Garewal HS. Antioxidants and Disease Prevention. New York: CRC Press, 1997, 19-26.
Glassman AH, Roose SP. Risks of antidepressants in the elderly: tricyclic antidepressants and arrhythmia-revising risks.
Gerontology 1994;40 Suppl 1:15-20.
Abstract: Unexpected events have occurred in cardiology over the last 4 years. A study by the Heart and Lung Institute of the National Institute of Health in the mid-1980s showed to great surprise that class I antiarrhythmic drugs given to patients with ventricular arrhythmias following myocardial infarction, instead of preventing deaths, actually increased the number of patients dying. Since then, a series of studies has consistently confirmed this original observation. The problem for psychiatry is that the tricyclic antidepressant (TCA) drugs are also class I antiarrhythmics. There is every reason to believe that a similar increased risk of death would exist with the TCAs. It is, therefore, important for psychiatrists to understand the cause and magnitude of this excess in deaths. Evidence to date would suggest that all class I compounds, despite being powerful antiarrhythmics under usual physiological conditions, become proarrhythmic under anoxic conditions. Such conditions would exist in ischaemic heart disease during angina and, particularly, myocardial infarction. How this might alter our use of TCAs and whether this happens with the selective serotonin reuptake inhibitors is discussed.
Kerns II W, Kline J, Ford MD. Blocker and calcium channel blocker toxicity.
Emerg Med Clinics of NA 1994;12:2:365-390.
Kishi T, Watanabe T, Folkers K. Bioenergetics in clinical medicine XV. Inhibition of coenzyme Q10-enzymes by clinically used adrenergic blockers of beta-receptors.
Res Commun Chem Pathol Pharmacol. 1977 May;17(1):157-164.
Abstract: Adrenergic blockers for beta-receptors were studied for inhibition of mitochrondrial CoQ10-enzymes. These enzymes are indispensable for the bioenegetics of the myocardium. Propranolol is frequently used to treat hypertension; in some patients, it depresses myocardial function as an adverse reaction. This side effect may be related to the inhibition by propranolol of CoQ10-enzymes of the myocardium. Timolol showed negligible inhibition of the CoQ10-enzyme, NADH-oxidase. Metoprolol was less inhibitory than propranolol. Five alprenolols showed inhibition which approached that of propranolol. The 1-isomer of alprenolol showed weak inhibition of another CoQ10-enzyme, succinoxidase, but the other beta-blockers were essentially non-inhibitory to this enzyme. The drug of choice is timolol, based on negligible inhibition of these bioenergetic enzymes of the heart, which correlates with its pharmacologically low cardiac depressant effects.
Kishi T, Okamoto T, Takahashi T, Goshima K, Yamagami T. Cardiostimulatory action of coenzyme Q homologues on cultured myocardial cells and their biochemical mechanisms.
Clin Investig 1993;71(8 Suppl):S71-75.
Abstract: The effect of coenzyme Q (CoQ) homologues on the beating of myocardial cells was investigated in cultured cell sheets from mouse fetuses and quail embryos. Myocardial cell sheets grown in Eagle's minimum essential medium with fetal bovine serum showed very weak and irregular beating when this serum was removed from the medium. However, the depressed beating rate and amplitude recovered almost completely within a few minutes by adding CoQ10 to the medium, and the effect of CoQ10 continued over 1 h. CoQ9 showed a cardiostimulatory effect similar to that of CoQ10, but CoQ8 and CoQ7 showed almost no effect. Short homologues (less than CoQ4) inhibited the beating of cell sheets. The cardiostimulatory effect of CoQ10 was not blocked by atenolol, a selective beta-blocker. In addition, CoQ10 stimulated the formation of ATP, not cAMP. CoQ0 and CoQ3 inhibited beating rates by inhibiting ATP formation. In conclusion, only native CoQ homologues having a nona- or decaprenyl group showed a cardiostimulatory effect on cultured myocardial cells, probably by stimulating mitochondrial ATP formation.
Laaksonen R, Ojala JP, Tikkanen MJ, Himberg JJ. Serum ubiquinone concentrations after short- and long-term treatment with HMG-CoA reductase inhibitors.
Eur J Clin Pharmacol 1994;46(4):313-317.
Abstract: Serum ubiquinone levels were studied during long- and short-term treatment with 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors in 17 men with primary non-familial hypercholesterolaemia. The serum ubiquinone levels were determined after the patients had received simvastatin (20-40 mg per day) for 4.7 years, after a 4 week treatment pause and again after they had resumed treatment with lovastatin (20-40 mg per day) for 12 weeks. During the treatment pause the average serum ubiquinone levels increased by 32%; resumption of treatment caused a reduction of 25%. The changes in the levels of ubiquinone and serum total cholesterol as well as those of ubiquinone and low-density lipoprotein cholesterol were closely parallel. This suggested that changes in serum ubiquinone reflected changes in cholesterol-containing serum lipoproteins which could serve as carrier vehicles for ubiquinone. After long-term simvastatin treatment and after short-term lovastatin treatment, average serum ubiquinone levels (1.16 and 1.22 mg.l-1, respectively) were similar to that observed in a group of apparently healthy middle-aged men (1.16 mg.l-1).
Landbo C, Almdal TP. [Interaction between warfarin and coenzyme Q10]. Ugeskr
Laeger. 1998 May 25;160(22):3226-3227. [Article in Danish]
Abstract: Coenzyme Q10 (Ubidecarenone) is marketed as a dietary supplement. Drug interaction between coenzyme Q10 and warfarin has previously been reported. In the present case, a 72-year-old female treated with warfarin showed less responsiveness to warfarin than previously. It appeared she had taken coenzyme Q10, and when this was stopped, her responsiveness to warfarin was the same as before. Coenzyme Q10 is chemically similar to K-vitamins, which may explain the interaction with warfarin. Patients in treatment with warfarin should be aware of the possible risk of treatment failure when taking coenzyme Q10. The need for questioning patients concerning not only medications but also use of dietary supplements and alternative medications is emphasised.
Loop RA, et al. Effects of ethanol, lovastatin and coenzyme Q10 treatment on antioxidants and TBA reactive material in liver of rats.
Mol Aspects Med. 1994;15 Suppl:s195-206.
Marz R. Medical Nutrition From Marz. Second Edition. Portland, OR. 1997.
Morisco C, Trimarco B, Condorelli M. Effect of coenzyme Q10 in patients with congestive heart failure: a long-term multicenter randomized study. Clin Invest
1993;71:S134-36.
Mortensen SA, Leth A, Agner E, Rohde M. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors.
Mol Aspects Med 1997;18 Suppl:S137-S144.
Abstract: Coenzyme Q10 (ubiquinone) the essential mitochondrial redox-component and endogenous antioxidant, packaged into the LDL + VLDL fractions of cholesterol, has been suggested as an important anti-risk factor for the development of atherosclerosis as explained by the oxidative theory. Forty-five hypercholesterolemic patients were randomized in a double-blind trial in order to be treated with increasing dosages of either lovastatin (20-80 mg/day) or pravastatin (10-40 mg/day) over a period of 18 weeks. Serum levels of coenzyme Q10 were measured parallel to the levels of cholesterol at baseline on placebo and diet and during active treatment. A dose-related significant decline of the total serum level of coenzyme Q10 was found in the pravastatin group from 1.27 +/- 0.34 at baseline to 1.02 +/- 0.31 mmol/l at the end of the study period (mean +/- S.D.), P < 0.01. After lovastatin therapy the decrease was significant as well and more pronounced, from 1.18 +/- 0.36 to 0.84 +/- 0.17 mmol/l, P < 0.001. Although HMG-CoA reductase inhibitors are safe and effective within a limited time horizon, continued vigilance of a possible adverse consequence from coenzyme Q10 lowering seems important during long-term therapy.
Mortensen SA, Vadhanavikit S, Baandrup U, Folkers K. Long-term coenzyme Q10 therapy: a major advance in the management of resistant myocardial failure.
Drug Exptl Clin Res 1985;11:581-593.
Mortensen SA. Perspectives on therapy of cardiovascular diseases with coenzyme Q10 (ubiquinone).
Clin Invest 1993;71:s116-123. (Review)
Palomaki A, Malminiemi K, Metsa-Ketela T. Enhanced oxidizability of ubiquinol and alpha-tocopherol during lovastatin treatment.
FEBS Lett 1997 Jun 30;410(2-3):254-258.
Abstract: A double-blinded, placebo-controlled cross-over trial was carried out with 27 hypercholesterolemic men with coronary heart disease. During the 6-week treatment period lovastatin (60 mg/day) decreased fasting serum LDL cholesterol by 45%, LDL phosphorus by 38% and apoB by 33%. Ubiquinol content diminished by 13% as measured per LDL phosphorus. When LDL was oxidized ex vivo with AMVN both LDL ubiquinol and alpha-tocopherol were exhausted faster after lovastatin treatment compared to placebo, by 24% (P < 0.005) and 36% (P < 0.0001), respectively. Lag time in copper-induced oxidation of LDL decreased by 7% (P < 0.01). This suggests diminished antioxidant-dependent resistance of LDL to the early phase of oxidative stress.
Palomaki A, Malminiemi K, Solakivi T, Malminiemi O. Ubiquinone supplementation during lovastatin treatment: effect on LDL oxidation ex vivo. J Lipid Res. 1998 Jul;39(7):1430-1437.
Abstract: A randomized, double-masked, placebo-controlled cross-over trial was carried out to evaluate whether ubiquinone supplementation (180 mg daily) corrects impaired defence against initiation of oxidation of low density lipoprotein (LDL) related to effective (60 mg daily) lovastatin treatment. Nineteen men with coronary heart disease and hypercholesterolemia received lovastatin with or without ubiquinone during 6-week periods after wash-out. The depletion times for LDL ubiquinol and reduced alpha-tocopherol were determined during oxidation induced by 2,2-azobis(2,4-dimethylvaleronitrile) (AMVN). Copper-mediated oxidation of LDL isolated by rapid density-gradient ultracentrifugation was used to measure the lag time to the propagation phase of conjugated diene formation. Compared to mere lovastatin therapy, ubiquinone supplementation lead to a 4.4-fold concentration of LDL ubiquinol (P < 0.0001). In spite of the 49% lengthening in depletion time (P < 0.0001) of LDL ubiquinol, the lag time in copper-mediated oxidation increased only by 5% (P = 0.02). Ubiquinone loading had no statistically significant effect on LDL alpha-tocopherol redox kinetics during high radical flux ex vivo. The faster depletion of LDL ubiquinol and shortened lag time in conjugated diene formation during high-dose lovastatin therapy may, at least partially, be restored with ubiquinone supplementation. However, the observed improvement in LDL antioxidative capacity was scarce, and the clinical relevance of ubiquinone supplementation during statin therapy remains open.
Pinto J, Huang YP, Pelliccione N, Rivlin RS. Cardiac sensitivity to the inhibitory effects of chlorpromazine, imipramine and amitriptyline upon formation of flavins.
Biochem Pharmacol 1982 Nov 1;31(21):3495-3499.
Abstract: Chlorpromazine, imipramine and amitriptyline, drugs structurally related to riboflavin, each inhibited the formation in vivo of flavin adenine dinucleotide (FAD) from riboflavin in rat heart at 2-5 mg/kg body weight, doses comparable on a weight basis to those used clinically. All three drugs inhibited FAD formation in heart within 5 hr after a single dose of 25 mg/kg. Chlorpromazine under these conditions also inhibited FAD formation in liver, cerebrum and cerebellum. A series of psychoactive agents structurally unrelated to riboflavin did not inhibit flavin formation in the organs tested. These findings indicate that the inhibitory effects of the drugs studied have organ specificity with respect to FAD formation.
Scahill L, Lynch KA. Tricyclic antidepressants: cardiac effects and clinical implications.
J Child Adolesc Psychiatr Nurs 1994 Jan-Mar;7(1):37-39.
Spigset O. Reduced effect of warfarin caused by ubidecarenone. Lancet 1994 Nov 12;344(8933):1372-1373. (Letter)
Werbach MR. Foundations of Nutritional Medicine. Tarzana, CA: Third Line Press, 1997. (Review).
Willis RA, Folkers K, Tucker JL, Ye CQ, Xia LJ, Tamagawa H. Lovastatin decreases coenzyme Q levels in rats.
Proc Natl Acad Sci U S A. 1990 Nov;87(22):8928-8930.