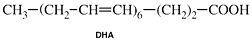
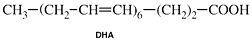
DHA
Common Name: DHAPlease read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
Alexander JW, Levy A, Custer D, Valente JF, Babcock G, Ogle CK, Schroeder TJ. Arginine, fish oil, and donor-specific transfusions independently improve cardiac allograft survival in rats given subtherapeutic doses of cyclosporin.
JPEN J Parenter Enteral Nutr 1998 May;22(3):152-155.
Assis SM, Monteiro JL, Seguro AC. L-Arginine and allopurinol protect against cyclosporine
nephrotoxicity. Transplantation 1997 Apr 27;63(8):1070-1073.
Abstract: The role of nitric oxide (NO) and oxygen free radicals in cyclosporine (CsA) nephrotoxicity was investigated using L-arginine, an NO substrate, and allopurinol, a xanthine oxidase inhibitor (involved in the formation of oxygen radicals) in an experimental model with Wistar rats. CsA, administered at 15 mg/kg/body weight (BW) subcutaneously for 10 days, caused a decrease in glomerular filtration rate, with inulin clearance of 0.33+/-0.04 vs. 1.11+/-0.06 ml/min/100 g BW (P<0.01 vs. control). L-Arginine, 1.5% in drinking water 5 days before and during CsA administration, partially protected the animals against this fall in glomerular filtration rate, with inulin clearance of 0.68+/-0.03 ml/min/100 g BW (P<0.01 vs. CsA). Allopurinol, at 10 mg/kg/BW by gavage, also had a protective action, with inulin clearance of 0.54+/-0.04 ml/min/100 g (P<0.01 vs. CsA). CsA caused an elevation in NO production, as assessed by urinary excretion of its metabolites, nitrite and nitrate (NO2 and NO3; 0.836+/-0.358 vs. 0.107+/-0.019 nmol/microg creatinine). NO production was as much as threefold higher in the L-arginine group (1.853+/-0.206 nmol/g creatinine). This CsA effect is probably related to its vasoconstrictive stimulus. Supplementation with L-arginine, which provides more substrate for NO formation, may enhance vasodilatation and consequently reduce the impairment of renal function. The protection provided by allopurinol may be related to the reduced formation of oxygen radicals, preventing the deleterious effects of lipid peroxidation.
Badalamenti S, Salerno F, Lorenzano E, Paone G, Como G, Finazzi S, Sacchetta AC, Rimola A, Graziani G, Galmarini D, et al. Renal effects of dietary supplementation with fish oil in cyclosporine-treated liver transplant recipients.
Hepatology 1995 Dec;22(6):1695-1671.
Abstract: Nephrotoxicity is the main untoward effect of cyclosporine (CsA) treatment. Experimental and clinical data suggest that dietary supplementation with fish oil may lessen cyclosporine nephrotoxicity, possibly by lowering renal thromboxane (Tx) production. We have studied the renal effects of a daily supplementation for 2 months of 12 g fish oil (18% C20:5 n-3 eicosapentaenoic acid [EPA] and 12% C22:6 n-3 docosahexanoic acid [DHA]) in a placebo-controlled (12 g corn oil), prospective, randomized, double-blind study of stable CsA-treated liver transplant recipients. Thirteen patients ingested corn oil capsules and 13 fish oil. Compliance with dietary regimen was confirmed by fatty acid chromatography that showed increased plasma concentrations of EPA (from 0.4 +/- 0.02% to 4.6 +/- 0.5%, P < .0001) and DHA (from 1.8 +/- 0.2% to 3.9 +/- 0.1%, P < .0001) in the fish oil group and increased plasma concentration of linoleic acid (C18:2 n-6) in the corn oil group (from 25 +/- 2% to 28.4 +/- 2%, P < .001). At the end of the 2 months of the study, in the fish oil group the effective renal plasma flow increased by 22% (P = .012), the glomerular filtration rate increased by 33% (P = .057), the renal blood flow increased by 17% (P = .024), and the calculated total renal vascular resistances decreased by 20% (P = .034). In contrast, none of these parameters changed in the corn oil group. The renal functional reserve determined during L-arginine infusion, plasma renin activity (PRA), and plasma aldosterone (PA) remained unchanged during the study in either group.
Barber MD, Ross JA, Preston T, Shenkin A, Fearon KC. Fish oil-enriched nutritional supplement attenuates progression of the acute-phase response in weight-losing patients with advanced pancreatic cancer.
J Nutr 1999 Jun;129(6):1120-1125.
Abstract: The presence of an acute-phase protein response (APPR) has been suggested to shorten survival and contribute to weight loss in patients with pancreatic cancer. Fatty acids derived from fish oil have been shown to alter proinflammatory cytokine production and acute-phase protein synthesis in vitro. The present study was designed to determine the effects of a fish oil-enriched nutritional supplement on the concentrations of a range of individual acute-phase proteins (APP) in patients with advanced pancreatic cancer. In a sequential series, 18 patients with pancreatic cancer received the supplement (providing 2 g eicosapentaenoic acid and 1 g docosahexaenoic acid/d) for 3 wk while another 18 received full supportive care alone. Six healthy subjects served as additional controls. Acute-phase proteins were measured before and after the 3-wk intervention period in cancer patients. At baseline, albumin, transferrin and pre-albumin were significantly reduced and fibrinogen, haptoglobin, alpha-1-acid glycoprotein, alpha-1-antitrypsin, ceruloplasmin and C-reactive protein (CRP) were significantly elevated in the cancer patients compared with healthy controls, reflecting their roles as negative and positive acute phase proteins, respectively. In the supplemented cancer group, the only significant change in APP concentrations over the 4-wk study period was an increase in transferrin. In the control cancer group there were further significant reductions in albumin, transferrin and pre-albumin, and a significant increase in CRP concentration. These results suggest that many positive and negative APP are altered in advanced pancreatic cancer. The APPR tends to progress in untreated patients but may be stabilized by the administration of a fish oil-enriched nutritional supplement. This may have implications for reducing wasting in such patients.
Bilo HJ, Homan van der Heide JJ, Gans RO, Donker AJ. Omega-3 polyunsaturated fatty acids in chronic renal insufficiency.
Nephron 1991;57(4):385-393. (Editorial; Review)
Conquer JA, Holub BJ. Supplementation with an algae source of docosahexaenoic acid increases (n-3) fatty acid status and alters selected risk factors for heart disease in vegetarian subjects.
J Nutr 1996;126:3032-3039.
Das UN, Madhavi N, Sravan Kumar G, Padma M, Sangeetha P. Can tumour cell drug resistance be reversed by essential fatty acids and their metabolites?
Prostaglandins Leukot Essent Fatty Acids 1998 Jan;58(1):39-54.
Tumour cell drug resistance is a major problem in cancer chemotherapy. Essential fatty acids have been shown to be cytotoxic to a variety of tumour cells in vitro. But, the effect of these fatty acids on tumour cell drug resistance has not been well characterized. Gamma-linolenic acid (GLA) of the n-6 series and eicosapentaenoic acid (EPA) of the n-3 series potentiated the cytotoxicity of anti-cancer drugs: vincristine, cis-platinum and doxorubicin on human cervical carcinoma (HeLa) cells in vitro. Alpha-linolenic acid (ALA), GLA, EPA and docosahexaenoic acid (DHA) enhanced the uptake of vincristine by HeLa cells. In addition, DHA, EPA, GLA and DGLA were found to be cytotoxic to both vincristine-sensitive (KB-3-1) and -resistant (KB-ChR-8-5) human cervical carcinoma cells in vitro. Pre-incubation of vincristine-resistant cells with sub-optimal doses of fatty acids enhanced the cytotoxic action of vincristine. GLA, DGLA, AA, EPA and DHA enhanced the uptake and inhibited the efflux of vincristine and thus, augmented the intracellular concentration of the anti-cancer drug(s). Fatty acid analysis of KB-3-1 and KB-ChR-8-5 cells showed that the latter contained low amounts of ALA, GLA, 22:5 n-3 and DHA in comparison to the vincristine-sensitive cells. The concentrations of GLA and DHA were increased 10-15 fold in the phospholipid, free fatty acid and ether lipid cellular lipid pools of GLA and DHA treated cells. These results coupled with the observation that various fatty acids can alter the activity of cell membrane bound enzymes such as sodium-potassium-ATPase and 5'-nucleotidase, levels of various anti-oxidants, p53 expression and the concentrations of protein kinase C suggest that essential fatty acids and their metabolites can reverse tumour cell drug-resistance at least in vitro.
Gibson RA, Neumann MA, Makrides M. Effect of dietary docosahexaenoic acid on brain composition and neural function in term infants.
Lipids 1996;31:177S-181S.
Horrobin DF. Schizophrenia as a prostaglandin deficiency disease. Lancet. 1977;1:936-937.
Horrobin DF, Glen AI, Vaddadi K. The membrane hypothesis of schizophrenia. Schizophr Res. 1994;13:195-207.
Horrocks LA, Yeo YK.Health Benefits of Docosahexanoic Acid (DHA).Pharmacol Res. 1999 Sep;40(3):211-225.
Abstract: Docosahexaenoic acid (DHA) is essential for the growth and functional development of the brain in infants. DHA is also required for maintenance of normal brain function in adults. The inclusion of plentiful DHA in the diet improves learning ability, whereas deficiencies of DHA are associated with deficits in learning. DHA is taken up by the brain in preference to other fatty acids. The turnover of DHA in the brain is very fast, more so than is generally realized. The visual acuity of healthy, full-term, formula-fed infants is increased when their formula includes DHA. During the last 50 years, many infants have been fed formula diets lacking DHA and other omega-3 fatty acids. DHA deficiencies are associated with foetal alcohol syndrome, attention deficit hyperactivity disorder, cystic fibrosis, phenylketonuria, unipolar depression, aggressive hostility, and adrenoleukodystrophy. Decreases in DHA in the brain are associated with cognitive decline during aging and with onset of sporadic Alzheimer disease. The leading cause of death in western nations is cardiovascular disease. Epidemiological studies have shown a strong correlation between fish consumption and reduction in sudden death from myocardial infarction. The reduction is approximately 50% with 200 mg day(-1)of DHA from fish. DHA is the active component in fish. Not only does fish oil reduce triglycerides in the blood and decrease thrombosis, but it also prevents cardiac arrhythmias. The association of DHA deficiency with depression is the reason for the robust positive correlation between depression and myocardial infarction. Patients with cardiovascular disease or Type II diabetes are often advised to adopt a low-fat diet with a high proportion of carbohydrate. A study with women shows that this type of diet increases plasma triglycerides and the severity of Type II diabetes and coronary heart disease. DHA is present in fatty fish (salmon, tuna, mackerel) and mother's milk. DHA is present at low levels in meat and eggs, but is not usually present in infant formulas. EPA, another long-chain n-3 fatty acid, is also present in fatty fish. The shorter chain n-3 fatty acid, alpha-linolenic acid, is not converted very well to DHA in man. These longchain n-3 fatty acids (also known as omega-3 fatty acids) are now becoming available in some foods, especially infant formula and eggs in Europe and Japan. Fish oil decreases the proliferation of tumour cells, whereas arachidonic acid, a longchain n-6 fatty acid, increases their proliferation. These opposite effects are also seen with inflammation, particularly with rheumatoid arthritis, and with asthma. DHA has a positive effect on diseases such as hypertension, arthritis, atherosclerosis, depression, adult-onset diabetes mellitus, myocardial infarction, thrombosis, and some cancers. Copyright 1999 Academic Press.
Iigo M, Nakagawa T, Ishikawa C, Iwahori Y, Asamoto M, Yazawa K, Araki E, Tsuda H. Inhibitory effects of docosahexaenoic acid on colon carcinoma 26 metastasis to the lung.
Br J Cancer 1997;75(5):650-655.
Abstract: Unsaturated fatty acids, including n-3 polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (C22:6, DHA) and eicosapentaenoic acid (C20:5, EPA), and a series of n-6 PUFAs were investigated for their anti-tumour and antimetastatic effects in a subcutaneous (s.c.) implanted highly metastatic colon carcinoma 26 (Co 26Lu) model. EPA and DHA exerted significant inhibitory effects on tumour growth at the implantation site and significantly decreased the numbers of lung metastatic nodules. Oleic acid also significantly inhibited lung metastatic nodules. Treatment with arachidonic acid showed a tendency for reduction in colonization. However, treatment with high doses of fatty acids, especially linoleic acid, increased the numbers of lung metastatic nodules. DHA and EPA only inhibited lung colonizations when administered together with the tumour cells, suggesting that their incorporation is necessary for an influence to be exerted. Chromatography confirmed that contents of fatty acids in both tumour tissues and plasma were indeed affected by the treatments. Tumour cells pretreated with fatty acids in vivo, in particular DHA, also showed a low potential for lung colony formation when transferred to new hosts. Thus, DHA treatment exerted marked antimetastatic activity associated with pronounced change in the fatty acid component of tumour cells. The results indicate that uptake of DHA into tumour cells results in altered tumour cell membrane characteristics and a decreased ability to metastasize.
Kooijmans-Coutinho MF, Rischen-Vos J, Hermans J, Arndt JW, van der Woude FJ. Dietary fish oil in renal transplant recipients treated with cyclosporin-A: no beneficial effects shown. J Am Soc Nephrol 1996 Mar;7(3):513-518.
Abstract: This study aimed to determine whether dietary supplementation with fish oil has a beneficial effect on graft function and the incidence of rejection in renal allograft recipients treated with cyclosporin A (CsA). Renal function, blood pressure, the incidence of acute rejection episodes, graft survival, and renal histology and immunochemistry were investigated. In a randomized, placebo-controlled, double-blind trial, groups of 25 recipients of primary cadaveric renal allografts who had been treated with CsA took fish oil (30% C20:5 omega-3 and 20% C22:6 omega-3) or coconut oil (63% C8:0 and 36% C10:0) at 6 g/day for 3 months. There were no differences between the two patient groups with regard to HLA matching, panel-reactive antibody titers, or the demographic characteristics of donors or recipients. The GFR and effective RPF were determined at 1, 3, and 12 months after transplantation by simultaneous measurement of (125I-)iothalamate and (131I) hippuran clearances. At 1 yr after transplantation, patients treated with fish oil showed better renal function than did the control patients, but this difference was not statistically significant. Blood pressure and antihypertensive drug use were similar in both groups. The number of rejection episodes was also similar, and renal histopathological and immunohistochemical studies showed no significant differences between the fish-oil group and the control patients. It is concluded that fish oil, at a dose of 6 g/day, has no beneficial effect after renal transplantation within the time scale of the study.
Leaf A, Weber PC. Cardiovascular effects of n-3 fatty acids. New Engl J Med 1988;318:549-557.
Makrides M, Neumann MA, Gibson RA. Is dietary docosahexaenoic acid essential for term infants?
Lipids 1996;31:115-119.
Malasanos TH, Stacpoole PW. Biological effects of omega-3 fatty acids in diabetes mellitus.
Diabetes Care 1991;14:1160-1179.
Mellor JE, Laugharne JDE, Peet M. Omega-3 fatty acid supplementation in schizophrenic patients.
Hum Psychopharmacol. 1996;11:39-46.
Pandalai PK, Pilat MJ, Yamazaki K, Naik H, Pienta KJ. The effects of omega-3 and omega-6 fatty acids on in vitro prostate cancer growth.
Anticancer Res 1996 Mar-Apr;16(2):815-820.
Dietary intake of essential fatty acids (EFA) may play a role in prostate cancer cell proliferation. Epidemiological studies have demonstrated that men whose dietary intake is high in omega-3 fatty acid (FA) composition have a lower incidence of clinical prostate cancer, suggesting that external factors such as diet may play an important role in development and growth of prostate cancer. Furthermore, in prostate cancer cell lines, omega-6 and omega-3 FAs have demonstrated promotional and inhibitory effects respectively. To investigate the effects of dietary fats on nontumorigenic prostate cell growth we conducted in vitro studies with human metastatic PC-3, LNCaP and TSU prostate cell lines, the rat metastatic Mat-Ly-Lu cell line and rat non-metastatic epithelial cell lines EPYP1, EPYP2 and EPYP3. Cell lines were treated with linoleic acid (LA), an omega-6 FA (n-6), as well as linolenic (LLA) and eicosapentaenoic (EPA) acids, which are both omega-3 FAs (n-3). All cell lines were treated with 10% and 0.5% serum supplemented media plus fatty acid for comparison. Our results demonstrate that linoleic acid(n-6) has promotional effects at doses of 1-100ng/ml in all cell lines with the exception of EPYPl. Experiments with linolenic acid (n-3) demonstrated consistent growth promotion in all cell lines examined with the exception of the EPYP2 cell line in which there was no significant effect. EPA had no effect in culture media supplemented with 10% serum, while in media containing 0.5% serum this FA demonstrated significant promotion in all human lines. Previous studies have indicated that EPA should inhibit human prostate cancer growth in vitro, however our results demonstrated promotion at low concentrations (lng/ml). At higher concentrations, EPA did inhibit prostate cell growth. These data indicate low levels of dietary fat, regardless of composition, may play a role in prostate cancer proliferation and could be an avenue for therapeutic intervention.
Peet M, Laugharne JDE, Mellor J, Ramchand CN. Essental fatty acid deficiency in erythrocyte membranes from chronic schizophrenic patients, and the clinical effects of dietary supplementation.
Prostaglandins Leukot Essent Fatty Acids. 1996;55:71-75.
Peet M, Laugharne JDE, Mellor J. Double-blind trial of n-3 fatty acid supplementation in the treatment of schizophrenia. Presented at the International Congress on Schizophrenia Research; April 1997; Colorado Springs, CO.
Schectman G, Kaul S, Kassebah AH. Effect of fish oil concentrate on lipoprotein composition in
NIDDM. Diabetes 1988;37:1567-1573.
Shahar E, Folsom AR, Melnick SL, Tockman MS, Comstock GW, Gennaro V, Higgins MW, Sorlie PD, Ko WJ, Szklo M. Dietary n-3 polyunsaturated fatty acids and smoking-related chronic obstructive pulmonary disease. Atherosclerosis Risk in Communities Study Investigators.
N Engl J Med. 1994 Jul 28;331(4):228-233.
Abstract: BACKGROUND. Fish contain n-3 polyunsaturated fatty acids, principally eicosapentaenoic acid and docosahexaenoic acid, which are known to interfere with the body's inflammatory response and may be of benefit in chronic inflammatory conditions. METHODS. We studied the relation between the dietary intake of n-3 fatty acids and chronic obstructive pulmonary disease (COPD) in 8960 current or former smokers participating in a population-based study of atherosclerosis. Intake of fatty acids was estimated with a dietary questionnaire. The presence of COPD was assessed by a questionnaire on respiratory symptoms and by spirometry. Three case definitions of COPD were used: symptoms of chronic bronchitis (667 subjects), physician-diagnosed emphysema reported by the subject (185 subjects), and spirometrically detected COPD (197 subjects). RESULTS. After control for pack-years of smoking, age, sex, race, height, weight, energy intake, and educational level, the combined intake of eicosapentaenoic acid and docosahexaenoic acid was inversely related to the risk of COPD in a quantity-dependent fashion. The adjusted odds ratio for the highest quartile of intake as compared with the lowest quartile was 0.66 for chronic bronchitis (95 percent confidence interval, 0.52 to 0.85; P < 0.001 for linear trend across the range of intake values), 0.31 for physician-diagnosed emphysema (95 percent confidence interval, 0.18 to 0.52; P for linear trend, 0.003), and 0.50 for spirometrically detected COPD (95 percent confidence interval, 0.32 to 0.79; P for linear trend, 0.007). CONCLUSIONS. A high dietary intake of n-3 fatty acids may protect cigarette smokers against COPD.
Shahar E, Boland LL, Folsom AR, Tockman MS, McGovern PG, Eckfeldt JH. Docosahexaenoic acid and smoking-related chronic obstructive pulmonary disease. The Atherosclerosis Risk in Communities Study Investigators.
Am J Respir Crit Care Med. 1999 Jun;159(6):1780-1785.
Abstract: If the inflammatory response to inhalation of cigarette smoke causes chronic obstructive pulmonary disease (COPD), suppression of that natural response might be beneficial. We hypothesized that a smoker's risk of developing COPD is inversely related to physiologic levels of two fatty acids that have antiinflammatory properties: eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6). The proportion of each fatty acid in plasma lipids was measured in 2,349 current or former smokers. COPD was identified and defined by clinical symptoms and/or spirometry. After adjustment for smoking exposure and other possible confounders, the prevalence odds of COPD were inversely related to the DHA (but not to the EPA) content of plasma lipid components in most of the models. For example, as compared with the first quartile of the DHA distribution, the prevalence odds ratios (ORs) for chronic bronchitis were 0.98, 0.88, and 0.69 for the second, third, and fourth quartiles, respectively (p for linear trend = 0.09). The corresponding ORs for COPD as defined spirometrically, were 0.65, 0.51, and 0.48 (p < 0. 001). Among 543 current heavy smokers, adjusted mean values of FEV1 (lowest to highest DHA quartile) were 2,706, 2,785, 2,801, and 2,854 ml. DHA may have a role in preventing or treating COPD and other chronic inflammatory conditions of the lung. Pilot testing of that hypothesis in experimental models seems warranted.
Simopoulos AP. Essential fatty acids in health and chronic disease.Am J Clin
Nutr. 1999 Sep;70(3 Suppl):560S-9S.
Abstract: Human beings evolved consuming a diet that contained about equal amounts of n-3 and n-6 essential fatty acids. Over the past 100-150 y there has been an enormous increase in the consumption of n-6 fatty acids due to the increased intake of vegetable oils from corn, sunflower seeds, safflower seeds, cottonseed, and soybeans. Today, in Western diets, the ratio of n-6 to n-3 fatty acids ranges from approximately 20-30:1 instead of the traditional range of 1-2:1. Studies indicate that a high intake of n-6 fatty acids shifts the physiologic state to one that is prothrombotic and proaggregatory, characterized by increases in blood viscosity, vasospasm, and vasoconstriction and decreases in bleeding time. n-3 Fatty acids, however, have antiinflammatory, antithrombotic, antiarrhythmic, hypolipidemic, and vasodilatory properties. These beneficial effects of n-3 fatty acids have been shown in the secondary prevention of coronary heart disease, hypertension, type 2 diabetes, and, in some patients with renal disease, rheumatoid arthritis, ulcerative colitis, Crohn disease, and chronic obstructive pulmonary disease. Most of the studies were carried out with fish oils [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)]. However, alpha-linolenic acid, found in green leafy vegetables, flaxseed, rapeseed, and walnuts, desaturates and elongates in the human body to EPA and DHA and by itself may have beneficial effects in health and in the control of chronic diseases.
Tisdale MJ. Inhibition of lipolysis and muscle protein degradation by EPA in cancer
cachexia. Nutrition 1996 Jan;12(1 Suppl):S31-33.
Abstract: Depletion of muscle and adipose tissue in cancer cachexia appears to arise not only from decreased food intake but also from the production of catabolic factors by certain tumours. Experiments with the cachexia-inducing MAC16 tumour in mice showed that when part of the carbohydrate calories were replaced by fish oil, host body weight loss was inhibited. The effect occurred without an alteration of either the total calorie consumption or nitrogen intake. Instead, one of the polyunsaturated fatty acids (PUFA) in fish oil, eicosapentaenoic acid (EPA), was found directly to inhibit tumour-induced lipolysis. The effect was structurally specific, as two related PUFA, docosahexaenoic acid (DHA) and gamma-linolenic acid (GLA), were without effect. The antilipolytic effect of EPA arose from an inhibition of the elevation of cyclic AMP in adipocytes in response to the lipid mobilizing factor. The increased protein degradation in the skeletal muscle of cachectic animals was also inhibited by EPA. This effect was due to the inhibition of the rise in muscle prostaglandin E2 in response to a tumour-produced proteolytic factor by EPA. Thus, reversal of cachexia by EPA in this mouse model results from its capacity to interfere with tumour-produced catabolic factors. Similar factors have been detected in human cancer cachexia.
Tisdale MJ. Wasting in cancer. J Nutr 1999 Jan;129(1S Suppl):243S-246S. (Review)
Progressive weight loss is a common feature of many types of cancer and is responsible not only for a poor quality of life and poor response to chemotherapy, but also a shorter survival time than is found in patients with comparable tumors without weight loss. Although anorexia is common, a decreased food intake alone is unable to account for the changes in body composition seen in cancer patients, and increasing nutrient intake is unable to reverse the wasting syndrome. Although energy expenditure is increased in some patients, cachexia can occur even with a normal energy expenditure. Various factors have been investigated as mediators of tissue wasting in cachexia. These include cytokines such as tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), interferon-gamma (IFN-gamma) and leukemia inhibitory factor (LIF), as well as tumor-derived factors such as lipid mobilizing factor (LMF) and protein mobilizing factor (PMF), which can directly mobilize fatty acids and amino acids from adipose tissue and skeletal muscle respectively. Induction of lipolysis by the cytokines is thought to result from an inhibition of lipoprotein lipase (LPL), although clinical studies provide no evidence for an inhibition of LPL in the adipose tissue of cancer patients. Instead there is an increased expression of hormone sensitive lipase, the enzyme activated by LMF. Protein degradation in cachexia is associated with an increased activity of the ATP-ubiquitin-proteasome pathway. The biological activity of both the LMF and PMF was shown to be attenuated by eicosapentaenoic acid (EPA). Clinical studies show that this polyunsaturated fatty acid is able to stabilize the rate of weight loss and adipose tissue and muscle mass in cachectic patients with unresectable pancreatic cancer. Knowledge of the mechanism of cancer cachexia should lead to the development of new therapeutic agents.
Werkman SH, Carlson SE. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until nine months.
Lipids 1996;31:91-97.
Wigmore SJ, Ross JA, Falconer JS, Plester CE, Tisdale MJ, Carter DC, Fearon KC. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer.
Nutrition 1996 Jan;12(1 Suppl):S27-30.
Abstract: Cachexia is common in patients with pancreatic cancer and has been associated with persistent activation of the hepatic acute phase response and increased energy expenditure. Fatty acids have been shown to have anticachectic effects in animal models and to reduce inflammatory mediators in healthy subjects and patients with chronic inflammatory disease. Eighteen patients with unresectable pancreatic cancer received dietary supplementation orally with fish oil capsules (1 g each) containing eicosapentaenoic acid 18% and docosahexaenoic acid 12%. Anthropometric measurement, body composition analysis, and measurement of resting energy expenditure and serum C-reactive protein were performed before and after supplementation with a median of 12 g/day of fish oil. Patients had a median weight loss of 2.9 kg/month (IQR 2-4.6) prior to supplementation. At a median of 3 months after commencement of fish oil supplementation, patients had a median weight gain of 0.3 kg/month (IQR 0-0.5) (p < 0.002). Changes in weight were accompanied by a temporary but significant reduction in acute phase protein production (p < 0.002) and by stabilisation of resting energy expenditure. This study suggests a component fish oil, perhaps EPA, merits further investigation in the treatment of cancer
cachexia.