
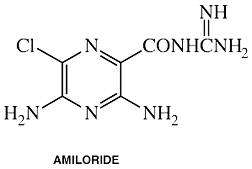
Amiloride
Brand Names: Midamor
Clinical Names: Amiloride
Summary
generic name: Amiloride
trade name: Midamor®
related drug: Amiloride/Hydrochlorothiazide
trade name: Moduretic®
type of drug: Potassium-sparing diuretic.
used to treat: Congestive heart failure, edema associated with kidney and liver diseases; hypertension.
overview of interactions:
• nutrient affected by drug: Potassium
• nutrient affected by drug: Sodium
• nutrients affected by drug: Food
• herb affecting drug performance: Glycyrrhiza glabra (Licorice)
Interactions
nutrient affected by drug: Magnesium
• mechanism: According to preliminary studies involving rats amiloride has a magnesium-sparing effect in addition to its potassium-sparing effect. Consequently there is the possibility that individuals who take a magnesium supplement while also taking amiloride could build up excessively high levels of magnesium. The concurrent use of hydrochlorothiazide and amiloride would make this accumulation unlikely given the magnesium-depleting action of hydrochlorothiazide.
(Devane J, Ryan MP. Br J Pharmacol. 1983 Aug;79(4):891-896; Devane J, Ryan MP.
Br J Pharmacol. 1981 Feb;72(2):285-289.)
• nutritional concern: Individuals taking amiloride should refrain from taking supplemental magnesium without first consulting their prescribing physician, pharmacist, or a healthcare professional experienced in nutritional therapies.
nutrient affected by drug: Potassium
• mechanism: Amiloride intentionally reduces urinary excretion of potassium. As a result of its role as a potassium-sparing diuretic, amiloride can produce a state of inappropriately elevated potassium levels.
• nutritional concerns: Individuals using potassium-sparing diuretics such as amiloride should limit their dietary intake of potassium to avoid excessive levels. Potassium supplements and potassium-containing salt substitutes, such as Lite Salt®, Morton's Salt Substitute and No Salt®, are designed for individuals suffering from potassium depletion due to other types of diuretics and should be avoided when taking potassium-sparing diuretics such as amiloride. For some individuals, foods with high potassium content may need to be limited. Several pieces of fruit per day may provide adequate potassium to elevate serum levels. Individuals taking amiloride should work with their prescribing physician to monitor potassium levels and modify their diet accordingly to avoid elevated potassium levels and associated problems.
(Stepan VM, et al. Eur J Gastroenterol Hepatol 1997 Oct;9(10):1001-1004.)
nutrient affected by drug: Sodium
• mechanism: The basic function of diuretics is to reduce the amount of water in the body. Therefore, by their very nature and intent diuretics, such as amiloride, increase the amount of sodium excreted in the urine.
• nutritional concerns: Since the reduction of sodium levels in the body is purposeful, supplementation to reduce lost sodium would be counterproductive. However, if dietary changes undertaken to restrict sodium intake are successful the dosage of diuretic medications will need to be reevaluated and possibly modified. Thus, individuals with hypertension who are using a diuretic such as amiloride while also strictly limiting their salt intake should work closely with their prescribing physician to monitor and revise their prescription based on changes in their blood pressure.
(Roe DA. 1989:146.)
nutrients affected by drug: Food
• mechanism: Amiloride reduces meal-stimulated colonic absorption through inhibition of both Na+ channels and Na+/H+ exchange in colonocytes. Constipation is also a common side effect of amiloride.
(Whang EE, et al. J Surg Res. 1996 Feb 1;60(2):303-306.)
• nutritional concerns: For best therapeutic effect and safest use, amiloride should be taken consistently at least two hours apart from food, preferably at the same time each day. Individuals concerned about the timing of their meals should consult with their prescribing doctor or pharmacist.
(Threlkeld DS, ed. Jul 1993.)
herb affecting drug performance: Glycyrrhiza glabra
(Licorice)
• mechanism: Licorice can offset the pharmacological effect of amiloride. 11 beta-hydroxysteroid dehydrogenase (11 beta-DH) is the enzyme that oxidizes cortisol to inactive cortisone and prevents cortisol from acting like a mineralocorticoid at the aldosterone receptor site in the kidney. Some kinds of licorice contain glycyrrhetic acid which inhibits the action of 11 beta-DH (e.g. in the kidney) and causes cortisol to behave like aldosterone. Thus, licorice consumption can induce a mineralocorticoid excess state, most likely due to an acquired inhibition of this key enzyme, decreased transformation of cortisol into cortisone, and resultant increased cortisol levels at the mineralocorticoid receptor. In states of 11 beta-DH deficiency such as the syndrome of apparent mineralocorticoid excess (AME) and licorice ingestion, cortisol acts as a potent mineralocorticoid. Thus, by acting to enhance aldosterone effects licorice would oppose the therapeutic intent of amiloride as an aldosterone antagonist or aldosterone-inhibiting agent. Furthermore, this increased mineralocorticoid action of cortisol can cause a drop in serum potassium and an increase in serum sodium concentration, together with a metabolic alkalosis, and lead to water retention, weight gain, and increased risk of hypertension.
(Miller LG. Arch Intern Med 1998 Nov 9;158(20):2200-2211; Lee YS, et al.
Clin Pharmacol Ther 1996 Jan;59(1):62-71; Pratesi C, et al. J Hypertens Suppl 1991 Dec;9(6):S274-275; Nanahoshi M.
Nippon Naibunpi Gakkai Zasshi 1967 Mar 20;42(12):1312-1319.)
• herbal concerns: The research cited above has focussed on concentrated extracts and intravenous forms of licorice. Common "licorice" candy usually contains no actual
Glycyrrhiza, other than perhaps a minute amount as flavoring. No solid conclusions can be drawn as to how much these findings relate to the use of licorice in the forms commonly used by practitioners of Western and Chinese herbal medicine. A product known as DGL (Deglycyrrhizinated Licorice) is available which retains the anti-inflammatory actions of whole licorice root without pseudo-aldosterone side effects. Individuals using amiloride should consult with their prescribing physician and/or a qualified practitioner of herbal medicine about the potential risks involved in using any form of licorice.

![]()
Please read the disclaimer concerning the intent and limitations of the information provided here.
Do not rely solely on the information in this article.
The information presented in Interactions is for informational and educational purposes only. It is based on scientific studies (human, animal, or in vitro), clinical experience, case reports, and/or traditional usage with sources as cited in each topic. The results reported may not necessarily occur in all individuals and different individuals with the same medical conditions with the same symptoms will often require differing treatments. For many of the conditions discussed, treatment with conventional medical therapies, including prescription drugs or over-the-counter medications, is also available. Consult your physician, an appropriately trained healthcare practitioner, and/or pharmacist for any health concern or medical problem before using any herbal products or nutritional supplements or before making any changes in prescribed medications and/or before attempting to independently treat a medical condition using supplements, herbs, remedies, or other forms of self-care.
References
Devane J, Ryan MP. Evidence for a magnesium-sparing action by amiloride during renal clearance studies in rats.
Br J Pharmacol. 1983 Aug;79(4):891-896.
Abstract: The potassium-sparing diuretic, amiloride, reduced the fractional excretion of magnesium in anaesthetized rats. Alterations in glomerular filtration rate (GFR), the filtered load of magnesium, arterial blood pressure, the status of the extracellular fluid volume, plasma aldosterone concentration and acid-base balance were not involved. It was concluded that amiloride exerted a magnesium-sparing effect by a direct renal action.
Devane J, Ryan MP. The effects of amiloride and triamterene on urinary magnesium excretion in conscious saline-loaded rats.
Br J Pharmacol. 1981 Feb;72(2):285-289.
Abstract: 1 The potassium-sparing diuretics, triamterene and amiloride, reduced urinary magnesium excretion in conscious saline-loaded rats. 2 Urinary magnesium-conservation was also detected when amiloride was used in combination with the potent 'loop-blocking' diuretic, frusemide.
Devane J, Ryan MP. Urinary magnesium excretion during amiloride administration in saline-loaded rats.
Br J Pharmacol. 1979 Nov;67(3):493P.
Gomez-Sanchez EP, Gomez-Sanchez CE. Effect of central amiloride infusion on mineralocorticoid hypertension.
Am J Physiol 1994 Nov;267(5 Pt 1):E754-758.
Abstract: There is strong evidence from different types of studies, including the discrete infusion of agonists and antagonists and ablation of specific brain areas or transmitter-type neurons, that mineralocorticoids, in excess, act in the brain to elevate blood pressure. Aldosterone enhances the entry of Na+ through amiloride-sensitive Na+ channels in some mineralocorticoid-sensitive transport epithelial cells. To define possible cellular mechanisms involved in central mineralocorticoid action, benzamil, an amiloride analogue with selective affinity for the Na+ channel, was continuously infused intracerebroventricularly in three mineralocorticoid-dependent hypertension models in Sprague-Dawley rats, the continuous subcutaneous infusion of aldosterone, the intracerebroventricular infusion of aldosterone, and the ingestion of carbenoxolone, a synthetic licorice analogue. The intracerebroventricular infusion of 0.3 and 0.5 micrograms/h of benzamil, doses that did not have an adverse effect on growth and that had no effect on the blood pressure when infused subcutaneously, prevented the increase in blood pressure in these models. The infusion of these levels of benzamil had no effect on urine volume even in those animals in which it prevented an increase in blood pressure. These data suggest that the central effects of mineralocorticoids on blood pressure are mediated, at least in part, by the effects of mineralocorticoids on amiloride-sensitive sodium transport.
Kleyman TR and Cragoe EJ Jr, The Mechanism of Action of Amiloride. Semin
Nephrol, 1988, 8(3):242-248.
Miller LG. Herbal medicinals: selected clinical considerations focusing on known or potential drug-herb interactions.
Arch Intern Med 1998 Nov 9;158(20):2200-2211. (Review)
Nanahoshi M. [Effect of glycyrrhizin on the action cortisone]. Nippon Naibunpi Gakkai Zasshi
1967 Mar 20;42(12):1312-1319. [Article in Japanese]
Pratesi C, Scali M, Zampollo V, Zennaro MC, De Lazzari P, Lewicka S, Vecsei P, Armanini D. Effects of licorice on urinary metabolites of cortisol and cortisone.
J Hypertens Suppl 1991 Dec;9(6):S274-275.
Roe DA. Diet and Drug Interactions. New York, Van Nostrand Reinhold, 1989: 146.
Stepan VM, Hammer HF, Krejs GJ. Hyperkalaemia and diarrhoea in a patient with surreptitious ingestion of potassium sparing diuretics.
Eur J Gastroenterol Hepatol 1997 Oct;9(10):1001-1004.
Abstract: We report a patient who presented with the unusual combination of chronic diarrhoea and hyperkalaemia. The patient was admitted to our hospital after repeated negative evaluations elsewhere including exploratory laparotomy. The patient had a long history of diarrhoea with hypokalaemia which was documented on several occasions in the past. Several months before admission to our hospital for evaluation of diarrhoea the patient developed hyperkalaemia. Her daily stool output reached 1200 g and her serum potassium was as high as 6.0 mmol/l. Extensive evaluation revealed surreptitious ingestion of the diuretics triamterene, hydrochlorothiazide and spironolactone as the cause of hyperkalaemia and diarrhoea. In addition, she had melanosis coli which was interpreted to be the consequence of surreptitious ingestion of anthraquinone-containing laxatives in the past although no current laxative intake could be proven. We postulate that diarrhoea in our patient was mainly due to the decreased sodium absorption in the small intestine and colon caused by diuretics. Serum aldosterone levels were more than eight times the upper limit of normal. Increased aldosterone levels presumably arose secondary to volume contraction and sodium chloride depletion, but presumably were not able to affect renal and colonic electrolyte transport because of blockage of mineralocorticoid receptors by spironolactone. Thus, the unusual combination of diarrhoea and hyperkalaemia resulted.
Threlkeld DS, ed. Diuretics and Cardiovasculars, Potassium-Sparing Diuretics, Spironolactone. In:
Facts and Comparisons Drug Information. St. Louis, MO: Facts and Comparisons, Jul 1993.
Whang EE, et al. Amiloride inhibits meal-stimulated colonic absorption.
J Surg Res. 1996 Feb 1;60(2):303-306.
Abstract: Meal-stimulated colonic absorption has recently been described, but the cellular transport mechanisms mediating this response are unknown. The purpose of this study was to determine the contribution of Na+ transport pathways to colonic proabsorption. Distal colonic Thiry-Vella loops were constructed in six dogs. Absorption was measured by infusing the loops with a physiological electrolyte solution containing [14C] polyethylene glycol as the impermeant marker. In the first set of experiments, the dose dependence of amiloride-induced inhibition of basal colonic absorption was determined. In the second set of experiments the effect of amiloride, which inhibits both Na+ channels and Na+/H+ exchange in colonocytes, on meal-stimulated colonic absorption was determined. Luminal amiloride inhibited basal colonic absorption in a dose-dependent manner, with significant reductions in Na+ absorption occurring with concentrations of 10(-2)M and higher. Infusion with 10(-3)M amiloride, a concentration that did not alter basal absorption, resulted in significant reductions in postprandial water, Na+, and Cl- absorption. These results suggest that meal-stimulated colonic absorption is mediated, at last in part, by transcellular Na+ absorptive pathways.