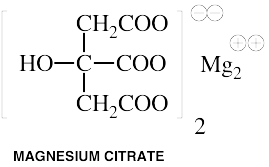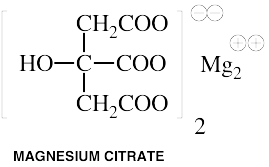
Magnesium
Common Names: Magnesium aspartate, Magnesium citrate, Magnesium fumarate, Magnesium malate, Magnesium succinate
Clinical Name: Magnesium
Summary
Magnesium
chemical name: Mg
forms: Magnesium aspartate, Magnesium citrate, Magnesium fumarate, Magnesium malate, Magnesium
succinate.
overview of interactions:
• nutrient affected by drug: Amiloride
• nutrient affected by drug: Aminoglycosides
• nutrient affecting drug performance: Calcium Channel Blockers
• nutrient affected by drug: Captopril
• Magnesium-based (or Aluminum-based) Antacids affecting drug performance: Ciprofloxacin
• nutrient affected by drug: Cisplatin
• nutrient affected by drug: Colchicine
• nutrient affected by drug: Corticosteroids, including Prednisone
• nutrient affected by drug and affecting drug toxicity: Cyclosporine
• nutrient affected by drug: Digoxin
• nutrient affected by drug: Loop Diuretics
and Thiazide Diuretics
• nutrient affected by drug: Gentamicin
• nutrient affected by drug: Lithium
• nutrient affected by drug: Neomycin
• nutrient affecting drug performance: Ranitidine
(Zantac®)
• nutrient affecting drug toxicity: Sulfonylureas
• nutrient affecting drug performance: Tetracyclines
• nutrient affecting drug performance: Warfarin
metabolism:
• The rate of magnesium absorption varies from as low as 24% to as high as 85%. Many factors regulate magnesium absorption. As the level of calcium intake goes down, the level of magnesium absorption goes up. A significant amount goes to the stomach for HCL production.
• Magnesium is very involved with ATP production via the Kreb's cycle.
• Glycolysis, adenyl cyclase and other reactions involving nerve impulse transmission also require high quantities of magnesium.
• High intakes of calcium, protein, vitamin D and alcohol all function to increase the magnesium requirement.
function:
• Magnesium regulates the absorption of calcium and is involved in the structural integrity of bones and teeth. If it is deficient in the bones, the bones may be dense but have poor trabecular integrity and thus be brittle. In both Finland and the Netherlands, there is a high ratio of calcium to magnesium intake (4 to 1) and the rate of osteoporosis is the highest in the world.
• Magnesium regulates the contractility of the heart muscle. It is concentrated 18x greater in the heart muscle than in the bloodstream. A decreased magnesium level in the heart muscle may predispose a person to coronary spasms. In areas where there is harder water (mainly due to magnesium), there is a much lower rate of heart disease.
• Magnesium has a relaxing effect on smooth muscle. It may be helpful in relaxing the smooth muscle of the bronchioles (improving asthma) and the arterioles (lowering blood pressure).
• It may relax uterine tissue (decreasing the cramping of dysmenorrhea), and also be useful in the treatment of angina and myocardial infarction.
• Magnesium decreases coagulation and acts as a calcium channel blocker. Thus, it helps the heart to pump more effectively.
• It is also one of the cofactors for delta 6 desaturase which is involved in the production of PGE1.
• Magnesium is necessary for the actions of PTH and 1,25 DHCC in bone calcium mobilization.
• Cofactor in many enzyme reactions (especially ATP reactions)
• Protein synthesis
• Role in neuromuscular transmitters
• Activates vitamin B-complex
dietary sources:
• High (200-400 mg/100 g food): nuts (almonds, cashews, Brazil), soybeans, brewer's yeast, buckwheat, wheat bran
• Good: Corn, peas, carrots, barley, oats, rye, wheat, rice bran, pecans, filberts, pistachios, black walnuts, green leafy vegetables (kale, endive, chard beet tops), celery, alfalfa, figs, apples, lemons, peaches, almonds, whole grains (millet, cornmeal, wheatgerm, barley, buckwheat, oats), tahini, sunflower seeds, brown rice, sesame seeds, black-eyed peas, lima beans, tofu, lentils, potato, sweet potato, peas, Brussel sprouts, broccoli, cauliflower, corn, avocado, dates, banana, blueberries, grape juice, cantaloupe, orange juice, milk
• The average intake of magnesium by so-called healthy adults in the U.S. and Western Europe ranges between 143-266 mg/day.
• High intakes of calcium, vitamin D, and protein increase the requirement for magnesium.
• Vitamin D, lactose and HCl all act to enhance absorption.
deficiency:
• Magnesium deficiency is very common in the West since magnesium is found predominantly in whole unprocessed foods.
• Deficiency symptoms include fatigue, irritability, weakness, muscle tightness or spasms, dysmenorrhea, high blood pressure, cardiomyopathy, nerve conduction problems, anorexia, insomnia, sugar cravings, poor nail growth, anxiety.
• Magnesium deficiency may be caused by any condition which increases loss or shifts the electrolyte balance, (such as renal disease) or diuretic therapy (such as antihypertensive medications).
• Malabsorption, hyperthyroidism, pancreatitis, kwashiorkor, diabetes, parathyroid gland disorders, poor intake, and diarrhea may also cause a deficiency.
• Serum magnesium is a very poor indicator of how much magnesium is actually in the tissues. For example, magnesium concentrates in the heart muscle at a 18x higher level than in the serum.
• Measuring white blood cell magnesium is a more sensitive indicator of tissue levels. An anionic magnesium measurement, recently pioneered by Drs. Burton and Bella Altura at Down-State University of New York in Brooklyn, appears to be a considerably more accurate indicator of tissue levels of magnesium than either WBC or RBC measurements.
known or potential therapeutic uses: Acute myocardial infarction, alcohol withdrawal, angina, anxiety, asthma, autism, cardiac arrhythmias, cardiomyopathy, cardiovascular disease, celiac disease, chronic fatigue syndrome (CFS), chronic obstructive pulmonary disease (COPD), congestive heart failure, constipation, diabetes mellitus, dysmenorrhea, eclampsia, eosinophilia-myalgia syndrome, fatigue, fibromyalgia, (acute) gastro-intestinal spasms or cramping, glaucoma, hearing loss, high blood pressure, high cholesterol, hyperactivity, hypoglycemia, intermittent claudication, kidney stones, lead toxicity, low HDL-cholesterol levels, migraine, mitral valve prolapse, muscle cramping, especially nocturnal, multiple sclerosis, osteoporosis, premenstrual syndrome, Raynaud’s disease, retinopathy, stroke, torticollis, toxemia of pregnancy.
maintenance dose: 500 mg per day.
• RDA:
Infants: 50-70 mg
Children: 150-300 mg
Adults: females: 280 mg per day; males: 350 mg per day
Pregnancy and lactation 350 mg per day
therapeutic dose: 500-1500 mg per day. Also used intravenously for many conditions.
side effects/toxicity:
• Hypermagnesia is rare but may result with decreased excretion, greatly increased absorption, or rarely with IM injection; toxicity results in depression of the central nervous system and possibly death
• Diarrhea is the most common adverse effect from magnesium. Excessive magnesium can actually lead to a magnesium deficiency if it causes chronic diarrhea. Magnesium also completes with calcium and can induce a calcium deficiency if calcium intake levels are already low. About 800 mg of elemental magnesium will generally cause loose stools but some people may be able to tolerate much higher doses. Different forms of magnesium, such as magnesium glycinate, may be tolerated differently as well. There is a slow release magnesium, Slo-mag, that may be helpful in elevating the intracellular levels of magnesium. Individuals with kidney failure must be cautious about magnesium supplementation since they may experience toxicity symptoms.
• Intravenous magnesium, because of its effect on smooth muscles, may cause hypotension along with dizziness and fainting. It may also cause respiratory depression or depletion of potassium.
• Intramuscular injections can often be painful and may cause a persistent lump if injection does not go deep enough to reach the muscle tissue. After the magnesium is loaded into the syringe a small amount of 2% lidocaine can be drawn into the tip of the syringe to ease the reaction. (Marz, 1997.)
contraindications: See interactions, most notably tetracyclines where magnesium interferes with drug metabolism.
Interactions
nutrient affected by drug: Amiloride
• mechanism: According to preliminary studies involving rats amiloride has a magnesium-sparing effect in addition to its potassium-sparing effect. Consequently there is the possibility that individuals who take a magnesium supplement while also taking amiloride could build up excessively high levels of magnesium. The concurrent use of hydrochlorothiazide and amiloride would make this accumulation unlikely given the magnesium-depleting action of hydrochlorothiazide.
(Devane J, Ryan MP. Br J Pharmacol. 1983 Aug;79(4):891-896; Devane J, Ryan MP.
Br J Pharmacol. 1981 Feb;72(2):285-289.)
• nutritional concern: Individuals taking amiloride should refrain from taking supplemental magnesium without first consulting their prescribing physician, pharmacist, or a healthcare professional experienced in nutritional therapies.
nutrient affected by drug: Aminoglycosides
• reports: Animal studies and case reports indicate that renal tubular damage due to aminoglycosides, such as gentamicin, can lead to hypomagnesemia combined with hypocalcemia, hypokalemia and alkalosis.
(Mazze RI, Cousins MJ. Br J Anaesth. 1973 Apr;45(4):394-398; Valdivieso A, et al.
Rev Med Chil. 1992 Aug;120(8):914-919; Kes P, et al. Magnes Trace Elem. 1990;9(1):54-60. Parsons PP, et al. Br J Pharmacol 1997 Oct;122(3):570-576.)
• research: Akbar et al have noted that hypomagnesemia may be especially common among children with cystic fibrosis who have a history of repeated use of aminoglycosides.
(Akbar A, et al. Acta Paediatr. 1999 Jul;88(7):783-785.)
• nutritional support: Individuals using aminoglycosides, especially on a repeated or chronic basis, should consult with their prescribing physician and/or a nutritionally oriented healthcare professional about nutritional support to restore normal levels of magnesium and these other important minerals. Patients undergoing extended treatment with aminoglycosides may need to have their doctor regularly monitor their kidney function along with magnesium and potassium status. Serum creatinine, BUN and creatinine clearance should be measured prior to initiating therapy and should be monitored throughout treatment. In this regard, many nutritionally-oriented practitioners find that testing magnesium levels in red blood cells is far more reliable than testing serum magnesium. Only after such assessment should supplementation with magnesium or potassium be undertaken and then only under close supervision by the prescribing physician.
Supplementation of magnesium in the dosage range of 300-500 mg per day is usually appropriate but should be done in consultation with the prescribing doctor or a nutritionally-oriented physician. Magnesium supplementation can be risky in patients with kidney damage and is usually contraindicated in such cases. It is also important to note that magnesium is needed to maintain intracellular potassium.
nutrient affecting drug performance: Calcium Channel Blockers
• research: Patients with variant angina often suffer from magnesium deficiency.
(Goto K, et al. Am J Cardiol. 1990 Mar 15;65(11):709-712.)
• nutritional synergy: Individuals taking calcium channel blockers, especially for variant angina, should consult with their prescribing physician and/or a healthcare provider trained in nutritional therapies about the potential benefits of supplementing with magnesium. Typical therapeutic dosages of magnesium are in the range of 250-350 mg per day for adults.
nutrient affected by drug: Captopril
• mechanism: Captopril increases lymphocyte magnesium levels, though possibly only in patients with pre-existing low levels.
(Lavin F, et al. Cardiology 1993;82(6):405-408; O'Keeffe S, et al. Cardiology 1992;80(2):100-105.)
Magnesium-based (or Aluminum-based) Antacids affecting drug performance: Ciprofloxacin
• mechanism: The absorption of ciprofloxacin is reduced by 50 to 90% in the presence of antacids containing magnesium and/or aluminum.
(Hoffken G, et al. Eur J Clin Microbiol. 1985 Jun;4(3):345; Gugler R, Allgayer H.
Clin Pharmacokinet 1990 Mar;18(3):210-219; Polk RE. Am J Med 1989 Nov 30;87(5A):76S-81S; Teixeira MH, et al.
J Chemother 1995 Apr;7(2):126-132; Mizuki Y, et al. J Antimicrob Chemother
1996 May;37 Suppl A:41-55.)
• nutritional concerns: Individuals taking ciprofloxacin should avoid using aluminium- or magnesium-based antacids without consulting the prescribing physician and/or a pharmacist.
nutrient affected by drug: Cisplatin
• mechanism: Cisplatin induces hypomagnesemia through its renal toxicity possibly by a direct injury to mechanisms of magnesium reabsorption in the ascending limb of the loop of Henle as well as the distal tubule. Consequently, cisplatin increases the urinary loss of magnesium. This drug-induced impairment of the renal tubules' ability to conserve magnesium may persist for months, or possibly years, after discontinuing use of the drug.
(Toffaletti J. Analyt Chem 1991 63(12):192R-194R; Lajer H, et al. Cancer Treat Rev. 1999 Feb;25(1):47-58; Koch Nogueira PC, et al. Pediatr Nephrol 1998 Sep;12(7):572-575.)
• nutritional support: One British study found higher serum magnesium concentration levels that children given intravenous magnesium before and after administration of cisplatin than in those give magnesium only after the cisplatin. The researchers concluded that magnesium supplements should be given to patients receiving cisplatin during the precisplatin hydration period to prevent hypomagnesemia.
(Kibirige MS, et al. Pediatr Hematol Oncol 1988;5(1):1-6.)
• nutrient affected by drug: Colchicine
• mechanism: Colchicine has been linked to impaired absorption of Magnesium.
(Roe DA. 1985, 159-160.)
• nutritional support: Individuals taking colchicine would most likely benefit from taking a high-potency multivitamin/mineral supplement to compensate for these interactions.
nutrient affected by drug: Corticosteroids, including Prednisone
• mechanism: Corticosteroids can contribute to depletion of magnesium.
(Holt GA. 1998, 83; Pronsky, Z. 1991, 60.)
• nutritional support: Individuals using corticosteroids for periods longer than two weeks should consult with their prescribing physician and/or a nutritionally trained healthcare professional about the potential need to supplement with magnesium to counter the depleting effects of the drug(s). A typical dose in such situations would be in the range of 300-400 mg of magnesium per day.
nutrient affected by drug and affecting drug toxicity: Cyclosporine
• mechanism: Cyclosporine has been linked to reduced serum levels of magnesium.
• adverse drug effects: This systemic depletion of magnesium produces a high risk of seizures due to cyclosporine-induced toxicity to the nervous system.
• testing: Individuals undergoing cyclosporine therapy should have their magnesium levels tested regularly. Nutritionally-oriented physicians generally find that monitoring red blood cell magnesium levels, rather than serum magnesium, is the most accurate method for diagnosing a deficiency.
• nutritional support: Magnesium supplementation prevents magnesium deficiency and subsequent neurotoxicity. In the event of cyclosporine-induced depletion, the prescribing physician should be consulted before starting any form of magnesium supplementation.
(Toffaletti J. Analyt Chem 1994 63(12):192R-194R; Pere AK, et al. Nephrol Dial Transplant
1998 Apr;13(4):904-910; Rob PM. Fortschr Med 1996 Apr 10;114(10):125-126; Thompson CB, et al.
Lancet 1984;ii:1116.)
nutrients affected by drug: Digoxin
• mechanism: Digoxin decreases intracellular magnesium, thereby causing increased urinary magnesium loss. Magnesium deficiencies induced by concommitant diuretic use are very common in individuals using digoxin. Hypomagnesemia may predispose to digitalis toxicity.
(Toffaletti J. Analyt Chem 1991 63(12):192R-194R; al-Ghamdi SM, et al. Am J Kidney Dis 1994 Nov;24(5):737-752.)
• clinical implications: Hypomagnesemia is known to produce a wide variety of clinical presentations, including neuromuscular irritability, cardiac arrhythmias, and increased sensitivity to digoxin. Magnesium deficiency also inhibits the therapeutic efficacy of digoxin in controlling atrial fibrillation. Refractory hypokalemia and hypocalcemia can be caused by concomitant hypomagnesemia and can be corrected with magnesium therapy.
(Toffaletti J. Analyt Chem 1991 63(12):192R-194R; Young IS, et al. Br J Clin
Pharmacol. 1991 Dec;32(6):717-721; Lewis R, et al. Br J Clin Pharmacol. 1991 Feb;31(2):200-203.)
• testing: Many physicians are aware of the need to monitor and prescribe for potassium depletion but do not consider the issue of magnesium deficiency unless serum levels fall below acceptable levels. Furthermore, many physicians experienced in nutritional assessment consider serum magnesium to be a very poor indicator of how much magnesium is actually in the tissues. Serum magnesium concentration is maintained within a narrow range by the kidney and small intestine since under conditions of magnesium deprivation both organs increase their fractional absorption of magnesium. If magnesium depletion continues, the bone store contributes by exchanging part of its content with extracellular fluid (ECF). The serum Mg can be normal in the presence of intracellular Mg depletion, and the occurrence of a low level usually indicates significant magnesium deficiency. Hypomagnesemia is frequently encountered in hospitalized patients and is seen most often in patients admitted to intensive care units. The detection of magnesium deficiency can be increased by measuring magnesium concentration in the urine or using the parenteral magnesium load test.
(al-Ghamdi SM, et al. Am J Kidney Dis 1994 Nov;24(5):737-752; Marz R. 1997.)
• nutritional support: Individuals taking digoxin will almost always benefit from supplementation of magnesium. Studies and clinical experience indicate that 300-500 mg of magnesium per day would be an appropriate dosage level for supplementing such patients. Anyone taking digoxin should consult the prescribing physician and/or a nutritionally-oriented healthcare professional regarding the issue of magnesium supplementation.
(Kinlay S, Buckley NA. J Toxicol Clin Toxicol 1995;33(1):55-59; Sueta CA, et al.
Magnes Res 1995 Dec;8(4):389-401.)
nutrient affected by drug: Loop Diuretics
and Thiazide Diuretics
• mechanism: By definition potassium-depleting diuretics increase potassium excretion and, in practice, they also usually deplete blood levels of magnesium. In turn, the drug-induced magnesium deficiency can contribute to further potassium depletion. Ultimately the relationship between these two patterns of depletion can be difficult to determine.
(Kroenke K, et al. Arch Intern Med 1987;147:1553-1556; Martin BJ, et al.
Arch Intern Med 1987 Oct;147(10):1768-1771.)
• adverse drug effects: A lack of magnesium interferes with healthy cardiac muscle function. This is especially important for patients on both diuretics and digitalis as they are more likely to develop arrythmias if not adequately supplemented with magnesium.
• testing: Serum levels of magnesium are not adequately sensitive to mild to moderate levels of depletion and thus are poor indicators of nutritional status.
• nutritional support: In practice, it is generally advisable for individuals taking any potassium-depleting diuretic, other than those with kidney failure, to supplement with both potassium and magnesium.
Supplementation of magnesium in the dosage range of 300-500 mg per day is usually appropriate but should be done in consultation with the prescribing doctor or a nutritionally-oriented physician. Magnesium supplementation can be risky in patients with kidney failure and usually contraindicated in such cases.
(Whang R, et al. Arch Intern Med 1992;152:40-45.)
nutrient affected by drug: Gentamicin
• mechanism: Research indicates that gentamicin can cause increased urinary magnesium loss.
• reports: Animal studies and case reports indicate that renal tubular damage due to aminoglycosides, such as gentamicin, can lead to hypokalemia combined with hypocalcemia, hypomagnesemia and alkalosis.
(Mazze RI, Cousins MJ. Br J Anaesth. 1973 Apr;45(4):394-398; Valdivieso A, et al.
Rev Med Chil. 1992 Aug;120(8):914-919; Kes P, et al. Magnes Trace Elem. 1990;9(1):54-60. Parsons PP, et al.
Br J Pharmacol 1997 Oct;122(3):570-576.)
• nutritional support: Even though there is no conclusive evidence showing the need for therapeutic supplementation, 300 mg per day would be a safe, protective dose of magnesium.
nutrient affected by drug: Lithium
• mechanism: Magnesium and lithium are chemically related. The consumption of lithium carbonate may cause high blood levels of magnesium.
(Herzberg L, Herzeberg B. J Nerv Ment Dis. 1977 Dec;165(6):423-426; Nielsen J.
Acta Psychiatr Scand 1964 40:190-196; Nielsen J. Acta Psychiatr Scand 1964;40:197-202.)
• nutritional concerns: Individuals taking lithium should inform their prescribing physican if they are also supplementing with magnesium. Likewise, the prescribing physician, a pharmacist, and/or a healthcare professional trained in nutrition should be consulted before starting any supplementation with magnesium, separately or as part of a multivitamin-mineral formulation.
nutrient affected by drug: Neomycin
• mechanism: Neomycin impairs magnesium absorption as a result of maldigestion when taken orally.
(Roe DA. 1985, 157-158.)
• nutritional support: Individuals taking neomycin internally for more than 2-3 days may benefit from taking supplemental magnesium at doses of 250-400 mg per day.
nutrient affecting drug performance: Ranitidine
(Zantac®)
• research: The concern regarding potential interaction between ranitidine and magnesium does not primarily originate with a drug-nutrient interaction per se. Instead, research on the interaction between magnesium hydroxide and ranitidine has found that some antacids reduce the bioavailability of the H2-receptor antagonists. This is particularly true when ranitidine is used at the same time as high doses of the relevant antacids. Bachmann et al found that among healthy subjects, i.e., ones who would not normally use the drug, a magnesium hydroxide/aluminum hydroxide antacid decreased ranitidine absorption by 20%-25% when the two substances were taken at the same time.
(Bachmann KA. et al. Scand J Gastroenterol Suppl 1994;206:14-19; Propst A, et al.
Arzneimittelforschung 1996 Jun;46(6):621-624.)
• nutritional support: In relation to antacid use, the potential for interaction can be reduced by taking the ranitidine at least two hours before or after any antacid containing aluminum or magnesium. There is concern that a multivitamin/mineral supplement containing magnesium could have the same effect, especially if the magnesium is in the form of magnesium hydroxide. In such cases, caution would advise taking the ranitidine at least two hours before or after the magnesium-containing supplement.
nutrient affecting drug toxicity: Sulfonylureas
• mechanism: Magnesium hydroxide administration may increase the risk of hypoglycemia from
sulfonylureas.
(Drug Evaluations Subscription. Vol. II, Section 10, Chapter 3, Winter, 1994; Schwanstecher M, et al.
Naunyn Schmiedebergs Arch Pharmacol 1991 Jan;343(1):83-89.)
nutrient affecting drug performance: Tetracyclines
• mechanism: Magnesium interferes with tetracycline absorption and reduces its effectiveness by chelating the drug. This interaction occurs not only with supplemental magnesium but also with many antacids, such as Pepto-Bismol, which contain aluminum magnesium hydroxide. Furthermore, in vitro studies indicate that chelates of magnesium and tetracycline may play a role in the toxicity of tetracycline.
(Machado FC, et al. J Inorg Biochem 1995 Nov 15;60(3):163-173; Drug Evaluation Subscription. Winter 1993.)
• nutritional concerns: Magnesium in the form of supplements should be avoided while using tetracycline. Tetracycline is best taken on an empty stomach, with a full glass of water, one hour before or two hours after ingestion of any supplements, food, or other drugs. Nevertheless, tetracycline and antacids are often used together in combination therapies for
Helicobacter pylori. Individuals taking tetracyline should only use magnesium supplements after consultation with the prescribing physician.
nutrient affecting drug performance: Warfarin
• mechanism: Mineral such as iron, magnesium, and zinc may bind with warfarin, thereby reducing their absorption and activity.
(Holt GA. 1998, 284.)
• nutritional concerns: While the chemistry of common mineral nutrients binding warfarin is well founded the clinical significance and frequency of occurrence of this interaction are uncertain. Individuals using warfarin should be aware of the possible risk of reduced effectiveness of treatment when taking supplements containing iron, magnesium and/or zinc. Usually taking these minerals at least two hours apart from the warfarin provides adequate protection from unwanted interference.


Please read the disclaimer concerning the intent
and limitations of the information provided here.
Do not rely solely on the information in this article.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
References
[No author given.] Drug Evaluations Subscription. Chicago: American Medical Association, Vol. II, Section 10, Chapter 3, Winter, 1994.
Akbar A, Rees JH, Nyamugunduru G, English MW, Spencer DA, Weller PH. Aminoglycoside-associated hypomagnesaemia in children with cystic fibrosis.
Acta Paediatr. 1999 Jul;88(7):783-785.
Abstract: Hypomagnesaemia in children with cystic fibrosis (CF) is under-recognized. We report a child with CF who developed significant hypomagnesaemia following intravenous (i.v.) treatment with aminoglycosides for exacerbations of Pseudomonas aeruginosa infection. Three additional cases have also been observed. Investigations in two patients have revealed excessive renal loss of magnesium. It is postulated that renal tubular damage secondary to the cumulative effects of repeated courses of aminoglycosides resulted in hypomagnesaemia, and we suggest screening for this problem by monitoring serum magnesium regularly in all patients with CF receiving multiple courses of aminoglycosides.
al-Ghamdi SM, Cameron EC, Sutton RA. Magnesium deficiency: pathophysiologic and clinical overview.
Am J Kidney Dis 1994 Nov;24(5):737-752. (Review)
Ariceta G, Rodriguez-Soriano J, Vallo A, Navajas A. Acute and chronic effects of cisplatin therapy on renal magnesium homeostasis.
Med Pediatr Oncol 1997 Jan;28(1):35-40.
Abstract: Although the acute renal toxicity of cisplatin has been well documented, long-term follow-up studies in cisplatin-treated children are scanty. We have evaluated the incidence and characteristics of both acute and chronic nephrotoxicity in 22 children (median age 8 years) treated with cisplatin as part of different chemotherapeutic protocols. All patients exhibited a significant and progressive decrease in plasma magnesium (Mg) values soon after cisplatin administration. Magnesiuria also increased immediately after therapy. Hypomagnesemia (plasma Mg < 1.4 mg/dl) occurred in 10 patients and it was dose-dependent. Minimal and mean cumulated doses inducing hypomagnesemia were 300 and 500 mg/m2, respectively. In 18 children we followed renal function prospectively for a mean time of 2.3 years after arrest of cisplatin therapy. Chronic hypomagnesemia and moderate elevation of plasma creatinine were observed in 6 children, hypocalciuria in 5 children, and hypokalemia in 1 child. Presence of hypomagnesemia was unrelated to the total dose received or the time elapsed since cisplatin therapy. Renal function studies, performed in the 6 children with chronic hypomagnesemia, revealed different degrees of impairment in Mg reabsorption. The functional characteristics of chronic cisplatin nephrotoxicity found in the present series-contrary to prior reports-are not comparable to those present in the inherited Gitelman's syndrome.
Bachmann KA, Sullivan TJ, Jauregui L, Reese J, Miller K, Levine LDrug interactions of H2-receptor antagonists.
Scand J Gastroenterol Suppl 1994;206:14-19.
Abstract: Three drug interactions of nizatidine and of other antisecretory agents were studied comparatively. First, the effects of nizatidine, cimetidine and ranitidine on the dispositional kinetics of theophylline were evaluated in chronic obstructive pulmonary disease (COPD) patients. Second, the effect of magnesium/aluminium hydroxide on the relative bioavailability of nizatidine, famotidine, cimetidine and ranitidine was evaluated in healthy volunteers. Finally, the effects of nizatidine and omeprazole on the dispositional kinetics of phenytoin were evaluated in healthy volunteers. Only cimetidine altered the steady-state kinetics of oral theophylline, slowing theophylline clearance by 25%. Each of the H2-receptor antagonists exhibited a modest decline in relative bioavailability when ingested with antacid. Antacid ingestion decreased the bioavailability of famotidine, ranitidine and cimetidine by 20-25%, and the bioavailability of nizatidine by 12%. Each of these effects was statistically significant. Finally, it was found that neither omeprazole nor nizatidine affected the single dose kinetics of phenytoin.
Barros LF, Chagas AC, da Luz PL, Pileggi F. Magnesium treatment of acute myocardial infarction: effects on necrosis in an occlusion/reperfusion dog model.
Intl J Cardiol. 1995 Jan 27 48(1):3-9.
Bianchetti MG, Kanaka C, Ridolfi-Luthy A, Wagner HP, Hirt A, Paunier L, Peheim E, Oetliker OH. Chronic renal magnesium loss, hypocalciuria and mild hypokalaemic metabolic alkalosis after cisplatin.
Pediatr Nephrol 1990 May;4(3):219-222.
Abstract: Renotubular handling of sodium, potassium (K) calcium (Ca), phosphate, hydrogen ions and glucose, and urinary concentrating ability were studied in three children (aged 8, 8.5, 11 years) with renal magnesium (Mg) loss, persisting for more than 2 years after discontinuation of cisplatin treatment for neuroblastoma. A group of healthy children served as controls. Besides renal Mg wasting, a clear-cut tendency towards reduced calciuria associated with normal or slightly elevated plasma Ca was observed. Plasma K tended to be low (3.4-3.7 mmol/l), and plasma chloride was normal. Plasma bicarbonate (HCO3) ranged from 24.9 to 27.8 mmol/l, and urinary pH was always less than 6.0, indicating a renal HCO3 threshold exceeding 24 mmol/l. Plasma creatinine levels, glucosuria and phosphaturia, and urinary concentrating capacity were adequate. Comparable features were found in three children (aged 4.5, 9, 13 years) with primary renotubular hypomagnesaemia-hypokalaemia and hypocalciuria. This study complements the picture of chronic cisplatin tubulopathy in childhood demonstrating that, apart from Mg wasting, a reduced Ca excretion, and a tendency to hypokalaemia and metabolic alkalosis exist. Thus cisplatin may induce renal functional damage identical to that found in primary renotubular hypomagnesaemia--hypokalaemia with hypocalciuria.
Buckley JE, Clark VL, Meyer TJ, Pearlman NW. Hypomagnesemia after cisplatin combination chemotherapy.
Arch Intern Med 1984 Dec;144(12):2347-2348.
Abstract: Sixty-six patients receiving a five-drug combination chemotherapy regimen containing low-dose cisplatin were studied for the presence of hypomagnesemia. Thirty-eight (76%) of 50 patients receiving treatment every four weeks became hypomagnesemic during treatment. The incidence increased with the cumulative cisplatin dose, ranging from 41% after a single course to 100% of patients receiving six cycles of therapy. The incidence seemed lower in patients receiving the combination with a greater interval (eight weeks v four weeks) between cycles. We report the incidence and severity of hypomagnesemia to be dose dependent. The cause of the higher incidence of hypomagnesemia observed in this series compared with others is unknown but may be related to an interaction of cisplatin with another drug contained in this regimen.
Cohen L, Kitzes R. Magnesium sulfate and digitalis-toxic arrhythmias. JAMA 1983 May 27;249(20):2808-2810.
Abstract: Seven patients with congestive heart failure receiving long-term diuretic treatment (more than three years) experienced idionodal tachycardia in the presence of apparently normal serum digoxin levels. Intravenous bolus administration of magnesium (Mg) sulfate, followed by intramuscular Mg repletion, abolished the digitalis-toxic arrhythmia. The finding of decreased lymphocyte Mg and potassium contents proved the existence of cellular Mg depletion associated with normal serum Mg levels in five patients and with hypomagnesemia in the other two. Decreased cellular Mg content with normal serum Mg level predisposes to digitalis-toxic arrhythmias.
Cohen L. Potassium replacement associated with the development of tetany in a patient with hypomagnesaemia.
Magnes Res 1993 Mar;6(1):43-45.
Abstract: A case of hypomagnesaemia secondary to cisplatin therapy and diarrhoea had concomitant hypokalaemia. Increasing the serum potassium level from 2.8 to 3.4 mmol/litre by potassium supplementation induced tetany. Hypokalaemia in the face of hypomagnesaemia may have a membrane-stabilizing effect and preserve excitability.
Cox IM, Campbell MJ, Dowson D. Red blood cell magnesium and chronic fatigue syndrome.
Lancet 1991 Mar 30;337(8744):757-760.
Abstract: The hypotheses that patients with chronic fatigue syndrome (CFS) have low red blood cell magnesium and that magnesium treatment would improve the wellbeing of such patients were tested in a case-control study and a randomised, double-blind, placebo-controlled trial, respectively. In the case-control study, 20 patients with CFS had lower red cell magnesium concentrations than did 20 healthy control subjects matched for age, sex, and social class (difference 0.1 mmol/l, 95% confidence interval [CI] 0.05 to 0.15). In the clinical trial, 32 patients with CFS were randomly allocated either to intramuscular magnesium sulphate every week for 6 weeks (15 patients) or to placebo (17). Patients treated with magnesium claimed to have improved energy levels, better emotional state, and less pain, as judged by changes in the Nottingham health profile. 12 of the 15 treated patients said that they had benefited from treatment, and in 7 patients energy score improved from the maximum to the minimum. By contrast, 3 of the 17 patients on placebo said that they felt better (difference 62%, 95% CI 35 to 90), and 1 patient had a better energy score. Red cell magnesium returned to normal in all patients on magnesium but in only 1 patient on placebo. The findings show that magnesium may have a role in CFS.
Deppermann KM, Lode H, Hoffken G, Tschink G, Kalz C, Koeppe P. Influence of ranitidine, pirenzepine, and aluminum magnesium hydroxide on the bioavailability of various antibiotics, including amoxicillin, cephalexin, doxycycline, and amoxicillin-clavulanic acid.
Antimicrob Agents Chemother 1989 Nov;33(11):1901-1907.
Devane J, Ryan MP. Evidence for a magnesium-sparing action by amiloride during renal clearance studies in rats.
Br J Pharmacol. 1983 Aug;79(4):891-896.
Abstract: The potassium-sparing diuretic, amiloride, reduced the fractional excretion of magnesium in anaesthetized rats. Alterations in glomerular filtration rate (GFR), the filtered load of magnesium, arterial blood pressure, the status of the extracellular fluid volume, plasma aldosterone concentration and acid-base balance were not involved. It was concluded that amiloride exerted a magnesium-sparing effect by a direct renal action.
Devane J, Ryan MP. The effects of amiloride and triamterene on urinary magnesium excretion in conscious saline-loaded rats.
Br J Pharmacol. 1981 Feb;72(2):285-289.
Abstract: 1 The potassium-sparing diuretics, triamterene and amiloride, reduced urinary magnesium excretion in conscious saline-loaded rats. 2 Urinary magnesium-conservation was also detected when amiloride was used in combination with the potent 'loop-blocking' diuretic, frusemide.
Devane J, Ryan MP. Urinary magnesium excretion during amiloride administration in saline-loaded rats.
Br J Pharmacol. 1979 Nov;67(3):493P.
Elisaf M, Milionis H, Siamopoulos KC. Hypomagnesemic hypokalemia and hypocalcemia: clinical and laboratory characteristics.
Miner Electrolyte Metab 1997;23(2):105-112
Elliott WC, Patchin DS. Effects and interactions of gentamicin, polyaspartic acid and diuretics on urine calcium concentration.
J Pharmacol Exp Ther 1995 Apr;273(1):280-284.
Abstract: Gentamicin causes isolated, reversible calciuria in rats by an unknown mechanism. We hypothesized that gentamicin calciuria is related to nonantibacterial properties that may interfere with transtubular calcium transport (calcium channel blockade, Na,K-ATPase inhibition or competition with calcium for binding to the brush-border membrane). The calciuric effect of gentamicin was compared to the calcium channel blockers lanthanum and cobalt, the Na,K-ATPase inhibitor ouabain and the polycation aprotinin (which competes with gentamicin for brush-border membrane binding). Although gentamicin 0.02 mmol/kg caused a 6-8-fold increase in urine calcium concentration, none of the other agents was calciuric. We also found that the calciuric effects of gentamicin and furosemide were additive, whereas the noncalciuric diuretic chlorothiazide had no effect on gentamicin calciuria. We also determined the effect of poly-L-aspartic acid (PAA), which binds gentamicin and prevents nephrotoxicity. PAA caused isolated calciuria similar in magnitude and character to gentamicin. However, PAA pretreatment decreased the magnitude of gentamicin calciuria to insignificance. PAA pretreatment did not prevent furosemide calciuresis. These results indicate that: 1) gentamicin and furosemide calciuria are caused by different mechanisms; 2) gentamicin calciuria is probably not mediated by calcium channel blockade, Na,K-ATPase inhibition or displacement of brush-border membrane-bound calcium; 3) gentamicin and PAA calciuria may reflect interference with intracellular events related to transtubular calcium transport.
Friemann EFJ, Lasch H-G, Friemann S, Golf S, Enzinger D, Temme H, Graef V, Katz N, Roka L, Tross H, Morr H. [Effect of magnesium treatment on chronic obstructive lung diseases.]
Medizinische Welt. 1991; Vol 42(4) (pp 311-315. [Article in German]
Galloe AM, Rasmussen HS, Jorgensen LN, Aurup P, Balslov S, Cintin C, Graudal N, McNair P. Influence of oral magnesium supplementation on cardiac events among survivors of an acute myocardial infarction.
BMJ. 1993 Sep 4;307(6904):585-587.
Galloe AM, Graudal NA. [Magnesium treatment of patients with acute myocardial infarction. A meta-analysis].
Ugeskrift for Laeger. 1995 Jan 23; 157(4):437-40. [Article in Danish]
Gantz NM. Magnesium and chronic fatigue. Lancet 1991;338:66. (Letter)
Garland HO, Phipps DJ, Harpur ES. Gentamicin-induced hypercalciuria in the rat: assessment of nephron site involved.
J Pharmacol Exp Ther. 1992 Oct;263(1):293-297.
Abstract: Two independent techniques were used in anesthetized rats in an attempt to locate the nephron site of the reduced tubular calcium reabsorption accompanying acute gentamicin infusion. The first technique was that of lithium clearance used to assess proximal sodium (and secondarily calcium) handling. Observations that lithium clearance was comparable in control and gentamicin-treated animals (1.83 +/- 0.39 vs. 1.46 +/- 0.14 ml.min-1 for first experimental period) suggests a lack of proximal effect of the drug. The second technique was that of tracer microinjection whereby superficial nephrons were injected with 45Ca and tubule calcium transport was assessed from the recovery of radioactivity in the final urine. 45Ca recovery values from distal microinjections were comparable in control and gentamicin-treated groups (81.1 +/- 2.0 vs. 77.7 +/- 4.6%). However, 45Ca recovery values from proximal microinjections were significantly higher in the gentamicin group (9.4 +/- 1.0 vs. 3.5 +/- 0.8%; P < .001). These data suggest that the effects of gentamicin on renal calcium handling are mediated at a nephron site proximal to the distal tubule (i.e., loop of Henle or proximal tubule itself). Closer examination of individual proximal micropuncture data may point to an effect occurring predominantly in the pars recta of the proximal tubule or loop of Henle. Taken together, the results of both parts of the present study suggest that the early physiological effects of gentamicin on the kidney occur in a different nephron segment from any subsequent nephrotoxicity.
Gaspar AZ, Gasser P, Flammer J. The influence of magnesium on visual field and peripheral vasospasm in glaucoma.
Ophthalmologica 1995;209:11-13.
Goto K, Yasue H, Okumura K, Matsuyama K, Kugiyama K, Miyagi H, Higashi T. Magnesium deficiency detected by intravenous loading test in variant angina pectoris.
Am J Cardiol. 1990 Mar 15;65(11):709-712.
Abstract: To study whether magnesium (Mg) deficiency is present in patients with variant angina, 24-hour Mg retention of low dose Mg (0.2 mEq/kg lean body weight) administered intravenously over 4 hours in 20 patients with variant angina was examined. No patient had received calcium antagonists before or during the study. All had attacks of chest pain associated with ST elevation on electrocardiograms. Twenty-one subjects without ischemic heart disease were studied as control subjects. Ten patients with variant angina were restudied 10 to 529 days (mean 235 +/- 30) after the treatment with calcium antagonists (diltiazem 120 to 240 or nifedipine 40 to 80 mg/day), which resulted in complete suppression of anginal attacks. The mean serum Mg concentrations in the patients with variant angina and the control subjects were 2.1 +/- 0.05 and 2.1 +/- 0.03 mg/dl, respectively (difference not significant). However, 24-hour Mg retention in the patients with variant angina was 60 +/- 5%, while that in the control subjects was 36 +/- 3% (p less than 0.001), suggesting that Mg deficiency is present in at least some patients with variant angina. The mean serum Mg concentrations before and after calcium antagonist treatment in 10 patients with variant angina were 2.1 +/- 0.09 and 2.1 +/- 0.07 mg/dl, respectively (difference not significant). However, 24-hour Mg retention decreased significantly (p less than 0.01) from 60 +/- 6 to 34 +/- 7% after the treatment. There is Mg deficiency in many patients with variant angina and it is corrected after treatment with calcium antagonists.
Gugler R, Allgayer H. Effects of antacids on the clinical pharmacokinetics of drugs. An update.
Clin Pharmacokinet 1990 Mar;18(3):210-219.
Abstract: Since a previous review by Hurwitz was published in 1977 a large number of reports on drug interactions with antacids have appeared, few of which are of clinical relevance. Tetracyclines form insoluble complex molecules by metal ion chelation with various antacids; tetracycline absorption may be decreased by more than 90% by this interaction. Of the new class of quinolone antibiotics, the absorption of ciprofloxacin and ofloxacin is reduced by 50 to 90% in the presence of aluminium- and magnesium hydroxide-containing antacids. In contrast to early work showing inhibition of the absorption of beta-adrenergic blocking drugs by antacids, subsequent studies did not confirm a reduction in the bioavailability of either atenolol or propranolol during antacid treatment; indeed, they showed an increase in the plasma concentrations of metoprolol when the drug was coadministered with an antacid. The bioavailability of captopril was significantly reduced in the presence of an antacid, and lower plasma concentrations of this angiotensin-converting enzyme inhibitor were accompanied by a reduction of its effect on the systolic blood pressure of the patients. The absorption of the cardiac glycosides digoxin and digitoxin is not inhibited by antacids to a significant degree, although earlier studies had shown a positive effect when the dissolution of the glycoside preparations was relatively poor. Antacids reduce the bioavailability of the H2-receptor antagonists cimetidine and ranitidine only when high antacid doses are used and when the drugs are administered simultaneously. The bioavailability of famotidine was not significantly altered by a potent antacid preparation, although a trend towards reduced absorption was observed. Iron absorption is significantly decreased in the presence of sodium bicarbonate and calcium carbonate, but is nearly complete when coadministered with aluminium-magnesium hydroxide. Nonsteroidal anti-inflammatory drugs such as naproxen, tenoxicam, ketoprofen, ibuprofen and piroxicam are not affected in their absorption by antacid treatment. Theophylline bioavailability is unchanged when the drug is given together with antacids, although its rate of absorption may be altered, leading to a reduction or an increase in the time of the occurrence of peak plasma drug concentrations.
Healy DP, Dansereau RJ, Dunn AB, Clendening CE, Mounts AW, Deepe GS Jr. Reduced tetracycline bioavailability caused by magnesium aluminum silicate in liquid formulations of bismuth subsalicylate.
Ann Pharmacother 1997 Dec;31(12):1460-1464.
Herzberg L, Herzeberg B. Mood change and magnesium. A possible interaction between magnesium and lithium?
J Nerv Ment Dis. 1977 Dec;165(6):423-426.
Abstract: Magnesium and lithium are chemically related. Magnesium is an essential ion in many enzyme systems and lithium is of value in the treatment of manic-depressive disease. A significant sex difference in mean plasma magnesium levels is reported in 44 depressed patients. It is suggested that further studies of magnesium metabolism are indicated and that they may provide a better understanding of manic-depressive disease and the mode of action of lithium.
Hinds G, Bell NP, McMaster D, McCluskey DR. Normal red cell magnesium concentrations and magnesium loading tests in patients with chronic fatigue syndrome. Ann Clin Biochem. 1994 Sep;31 ( Pt 5):459-461.
Abstract: Red blood cell magnesium concentrations were measured in samples from 89 patients who fulfilled the diagnostic criteria for chronic fatigue syndrome and the results compared to those found in an age and sex matched group selected from the normal population. No significant difference was found. Six patients were further investigated using a magnesium loading test to determine if there was any evidence of magnesium deficiency associated with this disorder. None was found. There is therefore no indication for the use of magnesium therapy in the management of this condition.
Hoffken G, Borner K, Glatzel PD, Koeppe P, Lode H. Reduced enteral absorption of ciprofloxacin in the presence of antacids.
Eur J Clin Microbiol. 1985 Jun;4(3):345. (Letter)
Holt GA. Food and Drug Interactions. Chicago: Precept Press, 1998.
Howard JM, Davies S, Hunnisett A. Magnesium and chronic fatigue syndrome.
Lancet 1992 Aug 15;340(8816):426.
Kawano Y, Matsuoka H, Takishita S, Omae T. Effects of magnesium supplementation in hypertensive patients.
Hypertension 1998 Aug;32(2):260-265.
Abstract: An increase in magnesium intake has been suggested to lower blood pressure (BP). However, the results of clinical studies are inconsistent. We studied the effects of magnesium supplementation on office, home, and ambulatory BPs in patients with essential hypertension. Sixty untreated or treated patients (34 men and 26 women, aged 33 to 74 years) with office BP >140/90 mm Hg were assigned to an 8-week magnesium supplementation period or an 8-week control period in a randomized crossover design. The subjects were given 20 mmol/d magnesium in the form of magnesium oxide during the intervention period. In the control period, office, home, and average 24-hour BPs (mean+/-SE) were 148.6+/-1.6/90.0+/-0.9, 136.4+/-1.3/86.8+/-0.9, and 133.7+/-1.3/81.0+/-0.8 mmHg, respectively. All of these BPs were significantly lower in the magnesium supplementation period than in the control period, although the differences were small (office, 3.7+/-1.3/1.7+/-0.7 mmHg; home, 2.0+/-0.8/1.4+/-0.6 mmHg; 24-hour, 2.5+/-1.0/1.4+/-0.6 mm Hg). Serum concentration and urinary excretion of magnesium increased significantly with magnesium supplementation. Changes in 24-hour systolic and diastolic BPs were correlated negatively with baseline BP or changes in serum magnesium concentration. These results indicate that magnesium supplementation lowers BP in hypertensive subjects and this effect is greater in subjects with higher BP. Our study supports the usefulness of increasing magnesium intake as a lifestyle modification in the management of hypertension, although its antihypertensive effect may be small.
Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992 Jun 4;69(18):108G-118G; disc. 118G-119G. (Review)
Kes P, Reiner Z. Symptomatic hypomagnesemia associated with gentamicin therapy.
Magnes Trace Elem. 1990;9(1):54-60.
Abstract: Seven patients (3 females, 4 males) developed symptomatic hypomagnesemia, hypocalcemia, and hypokalemia following gentamicin therapy. The excessive and inappropriate urinary excretion of magnesium and potassium in the presence of subnormal serum concentrations was noted. A significant correlation was found between the total cumulative dose of gentamicin and serum Mg concentration (r = 0.76, p less than 0.05), as well as between the renal wasting of Mg and the total cumulative dose of gentamicin administered (r = 0.89, p less than 0.01). The gentamicin-induced Mg depletion is a very rare but important complication which is most likely to occur when the drug is given to older patients in large doses over extended periods of time.
Kibirige MS, Morris-Jones PH, Addison GM. Prevention of cisplatin-induced hypomagnesemia.
Pediatr Hematol Oncol 1988;5(1):1-6.
Abstract: Twenty-eight children were treated for various cancers with protocols that included dichlorodiamine platinum (cisplatin). Sixteen children were given intravenous magnesium after the administration of cisplatin, and 12 were given intravenous magnesium before and after administration of cisplatin. Serum magnesium concentration levels were monitored before, during, and after the full course of treatment and found to be lower in the first group of patients than in the second group. We recommend that magnesium supplements be given to patients receiving cisplatin during the precisplatin hydration period to prevent hypomagnesemia.
Kinlay S, Buckley NA. Magnesium sulfate in the treatment of ventricular arrhythmias due to digoxin toxicity.
J Toxicol Clin Toxicol 1995;33(1):55-59.
Abstract: Although digoxin antibodies are the definitive treatment of cardiac arrhythmias due to digoxin toxicity, magnesium can also be effective especially with low serum magnesium levels. The case report describes a patient with digoxin toxicity, ventricular tachycardia and a slightly elevated serum magnesium. Two 10 mmol doses of intravenous magnesium sulfate were associated with a more stable junctional rhythm with bigeminy. Magnesium is known to suppress early after depolarizations, and in supraphysiological doses, may act as an indirect antagonist of digoxin at the sarcolemma Na(+)-K(+)-ATPase pump. Intravenous magnesium may be used to treat cardiac arrhythmias due to digoxin poisoning where there is likely to be a delay in the availability of digoxin antibodies, even in the presence of elevated serum magnesium.
Koch Nogueira PC, Hadj-Aissa A, Schell M, Dubourg L, Brunat-Mentigny M, Cochat P. Long-term nephrotoxicity of cisplatin, ifosfamide, and methotrexate in osteosarcoma. Pediatr Nephrol 1998 Sep;12(7):572-575.
Abstract: The acute renal effects of chemotherapy are known, but long-term nephrotoxicity has rarely been investigated. The aim of the present study was to assess long-term renal function in children and adolescents who received at-risk chemotherapy, including cisplatin, ifosfamide, and methotrexate, to treat an osteosarcoma. Renal function tests [creatinine clearance, microalbuminuria, and renal excretion of sodium, potassium, chloride, calcium, magnesium (Mg), phosphorus (P), and uric acid] were prospectively performed 5.4+/-2.2 (+/-SD) years after chemotherapy (total cumulative dose: methotrexate 41+/-31 g/m2, ifosfamide 39+/-14 g/m2, cisplatin 674+/-188 mg/m2) in 18 children and adolescents. The results were compared with 13 normal volunteers matched for age and sex. Creatinine clearance, which was greater than 80 ml/min per 1.73 m2 in all patients, correlated with the total dose of ifosfamide (r=0.55, P<0.05) and cisplatin (r=0.48, P<0.05). Microalbuminuria was noted in 4 patients. Hypomagnesemia was present in 4 and hypercalciuria in 3 patients; renal excretion of P, Mg, and uric acid was higher in patients than in controls. Glomerular function was not significantly altered and only mild tubular dysfunction was present. Since renal excretion of P and Mg were increased in patients compared with normal volunteers and hypercalciuria was occasionally seen, divalent ion disorders are the most-likely potential complications.
Kosek JC, Mazze RI, Cousins MJ. Nephrotoxicity of gentamicin. Lab Invest. 1974 Jan;30(1):48-57.
Kotsaki-Kovatsi VP, Koehler-Samouilidis G, Kovatsis A, Rozos G. Fluctuation of zinc, copper, magnesium and calcium concentrations in guinea pig tissues after administration of captopril (SQ 14225).
J Trace Elem Med Biol 1997 Apr;11(1):32-36.
Abstract: The effect of the administration of captopril on Zn (zinc), Cu (copper), Ca (calcium) and Mg (magnesium) concentrations in guinea pig tissues was studied. For nine weeks 2 mg captopril per kg b.w. were administered daily to adult male guinea pigs intraperitoneally. The concentrations of the studied metals were determined in several tissues. Captopril significantly decreased Zn concentration in liver, Cu concentration in liver, adrenals, jejunum, urine and hair and Mg concentrations in blood and urine. A significant increase was observed in testicular and epididymal Zn, in heart, epididymal and fecal Cu, in Mg concentration of lung, kidney, adrenals, jejunum, epididymis and hair and in Ca concentrations in brain, heart, lung, kidney, spleen and stomach. No significant changes were observed in the colon and the thigh bone concentrations of the various elements tested. In conclusion Captopril treatment can produce translocation and/or elimination of Zn, Cu, Mg and Ca ions in various tissues of guinea pigs.
Kroenke K, Wood DR, Hanley JF. The value of serum magnesium determination in hypertensive patients receiving diuretics. Arch Intern Med 1987;147:1553-1556.
Kupfer S, Kosovsky JD. Effects of cardiac Glycosides on renal tubular transport of calcium, magnesium inorganic phosphate and glucose in the dog.
J Clin Invest 1965 44:1132-1143.
Lajer H, Daugaard G. Cisplatin and hypomagnesemia. Cancer Treat Rev. 1999 Feb;25(1):47-58. (Review)
Abstract: Hypomagnesemia is a well known side-effect in patients receiving cisplatin-containing chemotherapy. Cisplatin induces hypomagnesemia through its renal toxicity possibly by a direct injury to mechanisms of magnesium reabsorption in the ascending limb of the loop of Henle as well as the distal tubule. Since the magnesium reabsorption process still remains to be fully characterized, the effect by cisplatin on this process remains uncertain. Hypomagnesemia is a frequent complication to chemotherapy with cisplatin affecting up to 90% of patients if no corrective measures are initiated. The clinical importance of this hypomagnesemia remains uncertain. Possible symptoms of hypomagnesemia can be impossible to distinguish from symptoms related to the underlying disease or the treatment with chemotherapy. Existing studies on how to supplement magnesium during treatment with cisplatin have focused mainly on the effect on serum magnesium values and erythrocyte magnesium concentrations but both parameters are poor indicators of body magnesium stores. As long as the relationship between hypomagnesemia and possible complications thereof remains poorly elucidated, it seems reasonable to try to avoid hypomagnesemia. The best results seem to be provided by adding magnesium to the pre- and posthydration fluids.
Landauer JA. Magnesium deficiency and digitalis toxicity. JAMA 1984 Feb 10;251(6):730. (Letter, Review)
Lavin F, O'Keeffe S, Grimes H, Finn J, Mannion A, Daly K. Effect of prolonged nifedipine or captopril therapy on lymphocyte magnesium and potassium levels in hypertension.
Cardiology 1993;82(6):405-408.
Abstract: The effect of prolonged treatment with calcium channel blockers on potassium and magnesium stores is uncertain. We measured lymphocyte and serum magnesium and potassium in 28 patients treated for hypertension for 6 months with nifedipine or captopril. There was no difference in serum or lymphocyte concentrations in the two groups compared to 45 healthy, normotensive controls. These results suggest that intracellular cation levels are maintained with prolonged therapy with calcium channel blockers.
Lewis R, Durnin C, McLay J, McEwen J, McDevitt DG. Magnesium deficiency may be an important determinant of ventricular ectopy in digitalised patients with chronic atrial fibrillation.
Br J Clin Pharmacol. 1991 Feb;31(2):200-203
Abstract: Digitalised patients with chronic atrial fibrillation (AF) have a high prevalence of ventricular premature beats (VPB); magnesium deficiency may be a contributory factor. We have used a magnesium loading-test to examine the relationship between ventricular ectopy and magnesium status in 14 digitalised patients with chronic AF. Among seven patients with infrequent VPB (less than 250 24 h-1; mean 107 24 h-1) mean magnesium retention was 10.1% and four subjects retained no significant quantities of magnesium, indicating magnesium repletion. Among the remaining seven patients, mean magnesium retention was significantly higher (33.1%, P less than 0.02) and all patients retained 20% or more of the load given. There was an overall relationship between Mg retention and numbers of VPB (rs = 0.54; P less than 0.05). Magnesium deficiency may be determinant of ventricular ectopy in digitalised patients with chronic AF.
Machado FC, Demicheli C, Garnier-Suillerot A, Beraldo H. Metal complexes of anhydrotetracycline. 2. Absorption and circular dichroism study of Mg(II), Al(III), and Fe(III) complexes. Possible influence of the Mg(II) complex on the toxic side effects of tetracycline.
J Inorg Biochem 1995 Nov 15;60(3):163-173.
Martin BJ, Milligan K. Diuretic-associated hypomagnesemia in the elderly.
Arch Intern Med 1987 Oct;147(10):1768-1771.
Abstract: Serum magnesium concentration was measured in 320 consecutive elderly patients (mean age, 81 years) receiving diuretic therapy at the time of hospital admission. When compared with serum concentrations of 250 elderly patients who were not taking diuretics at the time of hospital admission, only the group taking thiazide diuretics had a significantly reduced mean serum level. The 24-hour urine sampling from representative subgroups demonstrated impaired magnesium-conserving ability in hypomagnesemic subjects receiving loop and thiazide diuretic therapy. Patients taking therapy that included a potassium-sparing diuretic had no significant evidence of reduced magnesium-conserving ability. Dietary assessments of the study population revealed suboptimal magnesium intake in the diet.
Marz R. Medical Nutrition From Marz. Second Edition. Portland, OR. 1997.
McLean, R. Magnesium and its therapeutic uses: A review. Am J Med 1994 Jan;96(1):63-76. (Review)
Mizuki Y, Fujiwara I, Yamaguchi T. Pharmacokinetic interactions related to the chemical structures of fluoroquinolones.
J Antimicrob Chemother 1996 May;37 Suppl A:41-55.
Abstract: Fluoroquinolone derivatives interact with methylxanthines (theophylline, caffeine) and metallic ion-containing drugs to different degrees. The rat appears to be a suitable model for predicting such interactions in man. It has been possible to determine the relationship between the chemical structure of the fluoroquinolone and the magnitude of the interaction. Fluoroquinolones with a bulky substituent at the position 8, such as sparfloxacin, lomefloxacin and fieroxacin, are less prone to interact with theophylline than those without an 8-substituent, such as enoxacin. This substituent determines the planarity of the whole fluoroquinolone molecule and the interaction tends to be more significant for planar fluoroquinolones. Furthermore, a 4'-nitrogen atom in the 7-piperazinyl group is essential for the interaction to occur. The nitrogen atom is possibly the site that binds cytochrome P-450, which catalyses theophylline metabolism. The reduction in bioavailability of fluoroquinolones by concurrent administration of aluminium hydroxide is more striking for derivatives with fewer substituents on the essential structure and on the piperazinyl group, such as norfloxacin, ciprofloxacin and enoxacin. Substitution at the 5-position diminishes the interaction, which suggests that the 5-substituent may affect the formation and/or stability of unabsorbable chelate complex which is the probable cause of the interaction. These findings are potentially useful in designing fluoroquinolones less prone to drug interactions.
Morten and Rasmussen, et al. Magnesium in the treatment of MI. Drugs 46:347-59, 1993.
Abstract: Magnesium needs to be given IV immediately during an MI to work effectively. In 76 patients with MI there was a decrease mortality rate of 55% of the patients treated with magnesium IV.
Nielsen J. Magnesium-lithium studies. 1. Serum and erythrocyte magnesium in patients with manic states during lithium treatment.
Acta Psychiatr Scand 1964 40:190-196.
Nielsen J. Magnesium-lithium studies. 2. The effect of lithium on serum magnesium in rabbits.
Acta Psychiatr Scand 1964;40:197-202.
O'Keeffe S, Grimes H, Finn J, McMurrough P, Daly K. Effect of captopril therapy on lymphocyte potassium and magnesium concentrations in patients with congestive heart failure.
Cardiology 1992;80(2):100-105.
Abstract: Lymphocyte potassium and magnesium were measured before and 3 months after the introduction of captopril in 18 patients taking diuretics for congestive heart failure. Compared to 32 healthy controls, 9 patients who had been on potassium supplements plus frusemide had decreased baseline lymphocyte magnesium and potassium concentrations (p less than 0.01), in spite of similar plasma electrolyte levels. There was a significant (p less than 0.01) increase in both lymphocyte potassium and magnesium levels after 3 months' treatment with captopril and frusemide in these patients. Nine patients who had been taking a potassium-sparing combination diuretic also had an increase in lymphocyte magnesium (p less than 0.05) following the introduction of captopril. Increased intracellular potassium and magnesium may be one mechanism whereby angiotensin-converting enzyme inhibitors reduced arrhythmias and improve survival in patients with congestive heart failure.
O’Keefe JH Jr, Harris WS, Nelson J, Windsor SL. Effects of pravastatin with niacin or magnesium on lipid levels and postprandial lipemia. Am J Cardiol 1995;76:480-484.
Oto A. Magnesium treatment in acute myocardial infarction: an unresolved consensus.
Eur Heart J. 1999 Jan;20(2):86-88. (Review)
Palmieri GM, Thompson JS, Eliel LP. Modifications of plasma magnesium by thyrocalcitonin, parathyroid extract and cortisone.
Endocrinology 1969 Jun;84(6):1509-1511.
Parsons PP, Garland HO, Harpur ES, Old S. Acute gentamicin-induced hypercalciuria and hypermagnesiuria in the rat: dose-response relationship and role of renal tubular injury.
Br J Pharmacol 1997 Oct;122(3):570-576.
Abstract: 1. Standard renal clearance techniques were used to assess the dose-response relationship between acute gentamicin infusion and the magnitude of hypercalciuria and hypermagnesiuria in the anaesthetized Sprague-Dawley rat. Also investigated were whether these effects occurred independently of renal tubular cell injury. 2. Acute gentamicin infusion was associated with a significant hypercalciuria and hypermagnesiuria evident within 30 min of drug infusion. The magnitude of these responses was related to the dose of drug infused (0.14-1.12 mg kg(-1) min[-1]). Increased urinary electrolyte losses resulted from a decreased tubular reabsorption of calcium and magnesium. 3. A rapid dose-related increase in urinary N-acetyl-beta-D-glucosaminidase (NAG) excretion was also observed in response to gentamicin infusion. However, there was no evidence of renal tubular cell injury and no myeloid bodies were observed within the lysosomes of the proximal tubular cells. Gentamicin may thus interfere with the mechanisms for cellular uptake and intracellular processing of NAG causing increased NAG release into the tubular lumen. 4. The absence of changes in renal cellular morphology indicates that the excessive renal losses of calcium and magnesium were an effect of gentamicin per se and not the result of underlying renal tubular injury. The renal effects described in this paper were apparent after administration of relatively low total drug doses, and with plasma concentrations calculated to be within the clinical range. These findings suggest that disturbances of plasma electrolyte homeostasis could occur in the absence of overt renal injury in patients receiving aminoglycoside antibiotics.
Pere AK, Krogerus L, Mervaala EM, Laakso J, Karppanen H, Inkinen K, Pere P, Ahonen J, Vapaatalo H, Lindgren L. Detrimental effect of dietary sodium and beneficial effect of dietary magnesium on glomerular changes in cyclosporin-A-treated spontaneously hypertensive rats.
Nephrol Dial Transplant 1998 Apr;13(4):904-910.
Perticone F, Borelli D, Ceravolo R, Mattioli PL. Antiarrhythmic short-term protective magnesium treatment in ischemic dilated cardiomyopathy.
J Am Coll Nutr. 1990;9(5);492-499.
Polk RE. Drug-drug interactions with ciprofloxacin and other fluoroquinolones.
Am J Med 1989 Nov 30;87(5A):76S-81S.
Abstract: Early investigational trials with new quinolone antibiotics revealed two important drug-drug interactions: decreased fluoroquinolone absorption when co-administered with magnesium-aluminum antacids and inhibition of theophylline metabolism. Subsequent studies have investigated the mechanisms of these interactions. With respect to the effect of antacids, the absorption of all quinolones appears to be significantly reduced by antacids containing magnesium and/or aluminum, and concomitant administration must be avoided. Other cations, such as calcium, iron, and probably zinc, appear to interact in a similar manner. Chelation between the quinolone and cation is the most likely mechanism. With respect to the effect on theophylline metabolism, quinolones inhibit specific cytochrome P-450 isozymes responsible for metabolism of methylxanthines, although there are major differences between the quinolones. Enoxacin is the most potent inhibitor, followed by ciprofloxacin, pefloxacin, norfloxacin, and ofloxacin. Caffeine metabolism is also inhibited, although the clinical significance is uncertain. Case reports describe renal failure associated with concomitant administration of cyclosporine and ciprofloxacin, although controlled trials have not demonstrated an interaction. Enoxacin has little effect on warfarin metabolism, suggesting that other quinolones may not affect warfarin disposition. Case reports of central nervous system toxicity from administration of nonsteroidal anti-inflammatory agents and quinolones need confirmation. Patients should be monitored closely when potential interacting agents are used; it is probable that not all interactions have been identified.
Polk RE, Healy DP, Sahai J, Drwal L, Racht E. Effect of ferrous sulfate and multivitamins with zinc on absorption of ciprofloxacin in normal volunteers.
Antimicrob Agents Chemother 1989 Nov;33(11):1841-1844.
Abstract: Cations such as magnesium and aluminum significantly impair the absorption of ciprofloxacin. Twelve healthy adult male volunteers participated in this four-way crossover study to investigate the effects of ferrous sulfate and multivitamins with zinc on the absorption of ciprofloxacin. Doses of ciprofloxacin (500 mg) were given 7 days apart and after an overnight fast. Dose 1 was administered alone (regimen A). The subjects then received either a ferrous sulfate tablet (325 mg three times a day; regimen B) or a once-daily multivitamin with zinc (regimen C) for 7 days; dose 2 of ciprofloxacin was then given with the last dose of regimen B or C. Subjects were crossed over to the alternate regimen for 7 days, and dose 3 of ciprofloxacin was again administered with the last dose of regimen B or C. After a 7-day washout, dose 4 of ciprofloxacin was given (regimen D). Ciprofloxacin concentrations were determined by high-pressure liquid chromatography. The areas under the concentration-time curve (AUCs) of ciprofloxacin for regimens A and D were not significantly different (14.5 +/- 2.3 versus 15.7 +/- 2.8 micrograms.h/ml, mean +/- standard deviation). The AUCs for regimen B (5.4 +/- 1.7 micrograms.h/ml) and regimen C (11.3 +/- 2.4 micrograms.h/ml) were significantly different from the AUCs for regimens A and D. Peak concentrations of ciprofloxacin with regimen B were below the MIC for 90% of strains of many organisms normally considered susceptible. Ferrous sulfate and multivitamins with zinc significantly impaired the absorption of ciprofloxacin. The effect of ferrous sulfate is likely to be clinically significant; the responsible component of multivitamins with zinc requires additional study.
Propst A, Propst T, Judmaier G. Comparison of the effects of ranitidine effervescent tablets and magnesium hydroxide-aluminium oxide on intragastric acidity. A single-centre, randomised, open cross-over study.
Arzneimittelforschung 1996 Jun;46(6):621-624.
Abstract: In previous studies measuring intragastric pH in healthy volunteers it was shown that there was a faster onset of action with ranitidine (CAS 66357-35-5) 300 mg effervescent tablets (Zantac) compared to standard tablets. In a single-centre, randomised, open cross-over study the pH-values obtained over 6 h following the administration of one ranitidine 150 mg effervescent tablet were compared with those after aluminium oxide-magnesium hydroxide (algeldrate, CAS 1330-44-5, Al-Mg-hydroxide) 10 ml and placebo in healthy volunteers. 24 healthy male subjects between 19 and 32 years of age entered the study, 19 subjects were available for all three measurements. After an overnight fast, intragastric pH was monitored for 7 h using a glass electrode and a digital data recorder. The time in % during which the pH was > or = 3.5 and the area under the curve of the obtained pH-curves were compared. There was a highly statistically significant difference between ranitidine effervescent tablets versus Al-Mg-hydroxide and placebo whereas there was no such difference between Al-Mg-hydroxide and placebo. The onset of action of ranitidine effervescent tablets was almost immediate. It is concluded that there was a clear superiority of ranitidine effervescent tablets in healthy volunteers and it is suggested that pH-metry in patients with acidity-related diseases should be investigated for a better understanding of the function of effervescent tablets.
Pronsky Z. Powers and Moore's Food-Medications Interactions. Ninth Edition. Food-Medication Interactions. Pottstown, PA, 1991.
Rasmussen HS. Clinical intervention studies on magnesium in myocardial infarction.
Magnesium. 1989;8(5-6):316-325. (Review)
Rasmussen HS, Gronbaek M, Cintin C, Balslov S, Norregard P, McNair P. One-year death rate in 270 patients with suspected acute myocardial infarction, initially treated with intravenous magnesium or placebo.
Clin Cardiol. 1988 Jun;11(6):377-381.
Abstract: In a double-blind, placebo-controlled study, 273 patients with suspected acute myocardial infarction (AMI) were randomized to receive either 48-h magnesium (Mg) or placebo therapy intravenously, initiated immediately on admission to hospital. We describe the results from a 1-year survey in 270 of the patients, who were available for follow-up. Patients were equally divided: 135 received Mg and 135 received placebo. Mg treatment was associated with a marked reduction in 1-year death rate from 32% in the placebo group to 20% in the Mg group (p = 0.018). If only death from ischemic heart disease is considered, the figures were 28% in the placebo group as opposed to 15% in the Mg group (p = 0.006). This reduction was mainly due to a reduction in mortality during the initial 30 days after inclusion in the study (17% vs. 7%), after which the difference in mortality between the two groups did not reach statistical significance (18% vs. 15%, p = 0.56). The beneficial effect of Mg on mortality was partly linked to a reduced incidence of arrhythmias (27% vs. 16%), and partly to a reduced incidence of infarction (63% vs. 48%) during the initial hospitalization. However, factors unknown to us were also involved, as revealed by a remaining statistically significant partial regression coefficient, when sex, age, cardiovascular history, development of AMI, and development of arrhythmias were considered. It is concluded that intravenous Mg treatment is beneficial to patients with acute ischemic heart disease and should be adopted as part of the routine treatment of these patients.
Rob PM. [Magnesium deficiency after kidney transplantation and cyclosporine therapy].
Fortschr Med 1996 Apr 10;114(10):125-126. [Article in German]
Robinson C, Weigly E. Basic Nutrition and Diet Therapy. New York: MacMillan, 1984.
Roe DA. Diet and Drug Interactions. New York: Van Nostrand Reinhold, 1989.
Roe DA. Drug-induced Nutritional Deficiencies. 2nd ed. Westport, CT: Avi Publishing, 1985.
Roe DA. Risk factors in drug-induced nutritional deficiencies. In: Roe DA, Campbell T, eds.
Drugs and Nutrients: The Interactive Effects. New York: Marcel Decker, 1984: 505-523.
Ryan MP. Interrelationships of magnesium and potassium homeostasis. Miner Electrolyte Metab 1993;19(4-5):290-295.
Abstract: The interrelationships of magnesium (Mg) and potassium (K) homeostasis are reviewed. Evidence from clinical and experimental studies including whole animal and cell culture experiments indicate that (1) homeostasis of Mg and K are closely related in the whole organism, (2) deficiencies of Mg and K frequently co-exist with gastrointestinal and especially renal losses from diuretic and nephrotoxic drug treatment being mainly responsible, and (3) Mg is required for maintenance of normal cellular K. Evidence from many laboratories indicate that Mg has direct effects at a cellular level on K transport. These include effects on Na-K-ATPase, Na-K-Cl cotransport, K channels, charge screening and permeability effects on membranes. New data on positive correlations between Mg and K in cardiac tissue, skeletal muscle and lymphocytes from patients undergoing cardiopulmonary bypass are presented. Interrelationships in Mg and K in cardiac tissue have probably the greatest clinical significance in terms of arrhythmias, digoxin toxicity, and myocardial infarction. Future studies will be aimed at elucidating mechanisms of Mg-K interrelationships at a cellular level using new techniques with the ability to detect concentrations and modulations of free intracellular Mg.
Sadowski DC. Drug interactions with antacids. Mechanisms and clinical significance.
Drug Saf 1994 Dec;11(6):395-407.
Schwanstecher M, Loser S, Rietze I, Panten U. Phosphate and thiophosphate group donating adenine and guanine nucleotides inhibit glibenclamide binding to membranes from pancreatic islets.
Naunyn Schmiedebergs Arch Pharmacol 1991 Jan;343(1):83-89.
Abstract: In microsomes obtained from mouse pancreatic islets, the Mg complex of adenosine 5'-triphosphate (MgATP) increased the dissociation constant (KD) for binding of [3H]glibenclamide by sixfold. In the presence of Mg2+, not only ATP but also adenosine 5'-0-(3-thiotriphosphate) (ATP gamma S), adenosine 5'-diphosphate (ADP), guanosine 5'-triphosphate (GTP), guanosine 5'-diphosphate (GDP), guanosine 5'-0-(3-thiotriphosphate) (GTP gamma S) and guanosine 5'-0-(2-thiodiphosphate) (GDP beta S) inhibited binding of [3H]glibenclamide. These effects were not observed in the absence of Mg2+. Half maximally effective concentrations of the Mg complexes of ATP, ADP, ATP gamma S and GDP were 11.6, 19.0, 62.3 and 90.1 mumol/l, respectively. The non-hydrolyzable analogues adenosine 5'-(beta,gamma-imidotriphosphate) (AMP-PNP) and guanosine 5'-(beta,gamma-imidotriphosphate) (GMP-PNP) did not alter [3H]glibenclamide binding in the presence of Mg2+, MgADP acted much more slowly than MgATP and both MgADP and MgGDP did not inhibit [3H]glibenclamide binding when the concentrations of MgATP and MgGTP were kept low by the hexokinase reaction. Development of MgATP-induced inhibition of [3H]glibenclamide binding and dissociation of [3H]glibenclamide binding occurred at similar rates. However, the reversal of MgATP-induced inhibition of [3H]glibenclamide binding was slower than the association of [3H]glibenclamide with its binding site. Exogenous alkaline phosphatase accelerated the reversal of MgATP-induced inhibition of [3H]glibenclamide binding. MgATP enhanced displacement of [3H]glibenclamide binding by diazoxide. The data suggest that sulfonylureas and diazoxide exert their effects by interaction with the same binding site at the sulfonylurea receptor and that protein phosphorylation modulates the affinity of the receptor.
Schultes G.[High doses of magnesium in the treatment of angina pectoris.]
Fortschritte der Medizin. 1991; 109(35); 81. [Article in German]
Shaheen BE, Cornish LA. Magnesium in the treatment of acute myocardial infarction.Clinical Pharmacy. 1993 Aug;12(8):588-596. (Review)
Shechter M, Merz CN, Paul-Labrador M, Meisel SR, Rude RK, Molloy MD, Dwyer JH, Shah PK, Kaul S. Oral magnesium supplementation inhibits platelet-dependent thrombosis in patients with coronary artery disease.
Am J Cardiol. 1999 Jul 15;84(2):152-156.
Abstract: The use of magnesium in the treatment of acute myocardial infarction remains controversial despite preliminary experimental evidence that magnesium plays a beneficial role as a regulator of thrombosis. This study examines whether oral magnesium treatment inhibits platelet-dependent thrombosis (PDT) in patients with coronary artery disease (CAD). In a randomized prospective, double-blind, crossover, and placebo-controlled study, 42 patients with CAD (37 men, 5 women, mean age 68 +/- 9 years) on aspirin received either magnesium oxide tablets (800 to 1,200 mg/day) or placebo for 3 months (phase 1) followed by a 4-week wash-out period, and the crossover treatment for 3 months (phase 2). PDT, platelet aggregation, platelet P-selectin flow cytometry, monocyte tissue factor procoagulant activity (TF-PCA), and adhesion molecule density were assessed before and after each phase. PDT was evaluated by an ex vivo perfusion model using the Badimon chamber. Median PDT was significantly reduced by 35% in patients who received magnesium versus placebo (delta change from baseline -24 vs 26 mm2/mm; p = 0.02, respectively). There was no significant effect of magnesium treatment on platelet aggregation, P-selectin expression, monocyte TF-PCA, or adhesion molecules. Oral magnesium treatment inhibited PDT in patients with stable CAD. This effect appears to be independent of platelet aggregation or P-selectin expression, and is evident despite aspirin therapy. These findings suggest a potential mechanism whereby magnesium may beneficially alter outcomes in patients with CAD.
Starobrat-Hermelin B, Kozielec T. The effects of magnesium physiological supplementation on hyperactivity in children with attention deficit hyperactivity disorder (ADHD): Positive response to magnesium oral loading test.
Magnesium Res 1997 Jun;10(2):149-156.
Abstract: Children with ADHD are 'a group at risk' as far as their further emotional and social development and educational possibilities are concerned, and the consequences of the lack of an appropriate therapy appears to be serious. Some of these children do not respond to prevailing therapy methods. It is reported that dietetic factors can play a significant role in the etiology of ADHD syndrome, and magnesium deficiency can help in revealing hyperactivity in children. The aim of our work was to assess the influence of magnesium supplementation on hyperactivity in patients with ADHD. The examination comprised 50 hyperactive children, aged 7-12 years, who fulfilled DSM IV criteria for ADHD syndrome, with recognized deficiency of magnesium in the blood (blood serum and red blood cells) and in hair using atomic absorption spectroscopy. In the period of 6 months those examined regularly took magnesium preparations in a dose of about 200 mg/day. 30 of those examined with ADHD showed coexisting disorders specific to developmental age, and 20 of them showed disruptive behaviour. The control group consisted of 25 children with ADHD and magnesium deficiency, who were treated in a standard way, without magnesium preparations. 15 members of this group showed coexisting disorders specific for developmental age, and 10 members showed disruptive behaviour. Hyperactivity was assessed with the aid of psychometric scales: the Conners Rating Scale for Parents and Teachers, Wender's Scale of Behavior and the Quotient of Development to Freedom from Distractibility. In the group of children given 6 months of magnesium supplementation, independently of other mental disorders coexisting with hyperactivity, an increase in magnesium contents in hair and a significant decrease of hyperactivity of those examined has been achieved, compared to their clinical state before supplementation and compared to the control group which had not been treated with magnesium.
Sueta CA, Patterson JH, Adams KF Jr. Antiarrhythmic action of pharmacological administration of magnesium in heart failure: a critical review of new data.
Magnes Res 1995 Dec;8(4):389-401.
Abstract: Congestive heart failure is characterized by contractile dysfunction and frequent complex ventricular ectopy. Despite advances in therapy, mortality from heart failure is substantial, estimated at 10-80 percent per year, and sudden death is common. Magnesium is the second most common intracellular cation, strongly influences cardiac cell membrane function, and is an important catalyst of many enzymatic reactions in the myocyte. Epidemiological studies have implicated magnesium deficit in the genesis of sudden death. Patients with congestive heart failure are predisposed to magnesium deficit for many reasons, including neurohormonal activation, poor gastrointestinal absorption, and drug therapy. Hypomagnesaemia is common in these patients and has been linked to an increased frequency of complex ventricular ectopy. Several early, uncontrolled studies have suggested a beneficial effect of magnesium administration on ventricular arrhythmias in patients with congestive heart failure. Two recent randomized, double blind, placebo-controlled trials have shown that both intravenous and oral administration of magnesium chloride results in a significant reduction in the frequency and complexity of ventricular arrhythmias in patients with congestive heart failure. Magnesium administration is well tolerated and serious adverse effects are rare. The potential mechanisms of the antiarrhythmic action of magnesium and limitations of the available data are discussed. The evidence reviewed suggests that serum magnesium concentrations should be monitored and corrected in patients with congestive heart failure. Ventricular arrhythmias may respond to acute intravenous magnesium administration, which should be considered as early therapy. Further study is needed to define magnesium dose and the effect of concomitant potassium administration. A prospective clinical trial is warranted to determine the chronic effects of magnesium administration in patients with heart failure.
Suzuki T, Koizumi J, Moroji T, Shiraishi H, Hori T, Baba A, Kawai N, Tada K. Effects of long-term anticonvulsant therapy on copper, zinc, and magnesium in hair and serum of epileptics.
Biol Psychiatry 1992 Mar 15;31(6):571-581.
Taubert K, Keil G. [Pilot study on the use of magnesium in the treatment of migraine and stress headache.]
Zeitschrift fur Arztliche Fortbildung. 1991; 85(1-2);67-68.
Teixeira MH, Vilas-Boas LF, Gil VM, Teixeira F. Complexes of ciprofloxacin with metal ions contained in antacid drugs.
J Chemother 1995 Apr;7(2):126-132.
Abstract: Simultaneous administration of antacids containing magnesium or aluminium and ciprofloxacin or other quinolones decreases the gastrointestinal absorption of those antibacterial agents. Current speculation about the mechanism of this interaction has focused on drug-cation chelation. The present study was designed to detect the protonation in solutions and the formation of the complex species at the pH levels typical of the gastrointestinal tract. It involves the study of ciprofloxacin in aqueous solutions containing Al3+ and (or) Mg2+ by combining the results of potentiometric and spectroscopic (1H nuclear magnetic resonance) techniques. Calculations were only performed for data in the range 4.5 < pH < 5.5 (pH levels typical of gastrointestinal tract) and the results of both methods are made self-consistent, assuming an equilibrium model including complex species MHL, MLOH (where H2L denotes ciprofloxacin and M is Al3+ or Mg2+); their formation constants are given.
Teixeira F, Geraldes CF, Gil VM, Helena M, Teixeira SF. In vitro complexation of aluminum and magnesium by cimetidine and ranitidine. A nuclear magnetic resonance study.
Gastroenterol Clin Biol 1984 Nov;8(11):879-80. (Letter)
Thompson CB, et al. Association between cyclosporine neurotoxicity and hypomagnesemia.
Lancet 1984;ii:1116.
Toffaletti J. Electrolytes, divalent cations, and blood gases (magnesium).
Analyt Chem 1991 63(12):192R-194R.
Trovato A, Nuhlicek DN, Midtling JE. Drug-nutrient interactions. Am Fam Physician 1991 Nov;44(5):1651-1658.(Review)
USDA. Composition of Foods. USDA Handbook #8. Washington DC, ARS, USDA, 1976-1986.
Valdivieso A, Mardones JM, Loyola MS, Cubillos AM. [Hypomagnesemia associated with hypokalemia, hyponatremia and metabolic alkalosis. Possible complication of gentamycin therapy].
Rev Med Chil. 1992 Aug;120(8):914-919. [Article in Spanish]
Abstract: Hypomagnesemia is a serious abnormality with different causes and usually associated to other disorders of electrolyte metabolism. We report a female patient developing hypomagnesemia after administration of gentamycin. This was associated to severe hypokalemia, hyponatremia and metabolic alkalosis. Possible pathogenetic mechanisms and therapeutic measures are discussed.
Watkins DW, Khalafi R, Cassidy MM, Vahouny GV. Alterations in calcium, magnesium, iron, and zinc metabolism by dietary cholestyramine.
Dig Dis Sci 1985 May;30(5):477-482.
Abstract: Cholestyramine is an effective drug for the reduction of plasma cholesterol because of its ability to sequester intestinal bile acids. Since metabolic alterations, including diminished intestinal absorption of vitamin D and osteomalacia have been reported with long-term use of this resin, the influence of cholestyramine on dietary balance of four mineral elements has been investigated. Wistar-strain rats were fed either a 2% cholestyramine or control diet for one month. Dietary intakes and fecal and urinary excretions of calcium, magnesium, iron, and zinc were determined using atomic absorption spectrophotometry during three, 3-day balance periods. Cholestyramine-fed rats had a net negative balance for calcium and a lower net positive balance for magnesium, iron, and zinc than the controls. Other effects of cholestyramine were an increased urinary excretion of calcium and magnesium, a decreased urinary zinc, and an alkalinization of urine. Blood and tissue cation content was unchanged except for a reduction in serum magnesium with resin feeding. Alterations in calcium, magnesium, and zinc metabolism might be explained by inadequate vitamin D absorption from the intestine followed by an increased secretion of parathyroid hormone. A diminished iron absorption due to resin binding could account for the reported disturbance in iron balance.
Weisinger JR, Bellorin-font, E. Magnesium and phosphorus. Lancet 1998 Aug 1;352(9125):391-396. (Review)
Werbach MR. Foundations of Nutritional Medicine. Tarzana, CA: Third Line Press, 1997. (Review).
Whang R, Oei TO, Watanabe A. Frequency of hypomagnesemia in hospitalized patients receiving digitalis.
Arch Intern Med 1985 Apr;145(4):655-656.
Abstract: We examined the frequency of hypokalemia and hypomagnesemia in patients receiving digitalis. Serum sodium, magnesium, and potassium levels were determined in 136 serum samples sent to the laboratory for digoxin assay. Hyponatremia (less than or equal to 130 mEq/L) occurred most frequently (21%), followed by hypomagnesemia (less than or equal to 1.25 mEq/L) in 19%, hypokalemia (less than or equal to 3.5 mEq/L) in 9%, and hypermagnesemia (greater than or equal to 2.25 mEq/L) in 7%. The twofold frequency of hypomagnesemia (19%) contrasted with hypokalemia (9%) indicates that clinicians are more attuned to avoiding hypokalemia than hypomagnesemia in patients receiving digitalis. Because hypokalemia and/or hypomagnesemia may contribute to the toxic effects of digitalis, ourobservation suggests that hypomagnesemia may be a more frequent contributor than hypokalemia to induction of toxic reactions to digitalis. Routine serum magnesium determination in patients receiving digitalis, who often are also receiving potent diuretics, may assist in identifying additional patients at risk for the toxic effects of digitalis.
Whang R, Whang DD, Ryan MP. Refractory potassium repletion-a consequence of magnesium deficiency.
Arch Intern Med 1992;152:40-45.
Abstract: Experimental and clinical observations support the view that uncorrected magnesium (Mg) deficiency impairs repletion of cellular potassium (K). This is consistent with the observed close association between K and Mg depletion. Concomitant Mg deficiency in K-depleted patients ranges from 38% to 42%. Refractory K repletion due to unrecognized concurrent Mg deficiency can be clinically perplexing. Refractory K repletion as a consequence of Mg deficiency may be operative in patients with congestive failure, digitalis toxicity, cisplatin therapy, and in patients receiving potent loop diuretics. Therefore, we recommend that: (1) serum Mg be routinely assessed in any patients in whom serum electrolytes are necessary for clinical management and (2) until serum Mg is routinely performed consideration should be given to treating hypokalemic patients with both Mg as well as K to avoid the problem of refractory K repletion due to coexisting Mg deficiency.
Young IS, Goh EM, McKillop UH, Stanford CF, Nicholls DP, Trimble ER. Magnesium status and digoxin toxicity.
Br J Clin Pharmacol. 1991 Dec;32(6):717-721.
Abstract: 1. Eighty-one hospital patients receiving digoxin were separated into groups with and without digoxin toxicity using clinical criteria. Serum digoxin, sodium, potassium, calcium, creatinine, magnesium and monocyte magnesium concentrations were compared. 2. Subjects with digoxin toxicity had impaired colour vision (P less than 0.0001, Farnsworth-Munsell 100 hue test) and increased digoxin levels (1.89 (1.56-2.21) vs 1.34 (1.20-1.47) nmol l-1, P less than 0.01) (mean (95% confidence limits], though there was considerable overlap between two groups. 3. Subjects with digoxin toxicity had lower levels of serum magnesium (0.80 (0.76-0.84) vs 0.88 (0.85-0.91) mmol l-1, P less than 0.01) and monocyte magnesium (6.40 (5.65-7.16) vs 8.76 (7.81-9.71) mg g-1 DNA, P less than 0.01), but there were no significant differences in other biochemical parameters. A greater proportion of toxic subjects were receiving concomitant diuretic therapy (20/21 vs 37/60, P less than 0.05). 4. Magnesium deficiency was the most frequently identified significant electrolyte disturbance in relation to digoxin toxicity. In the presence of magnesium deficiency digoxin toxicity developed at relatively low serum digoxin concentrations.