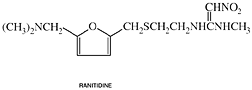

Ranitidine
Brand Names: Zantac, Zantac 75
Clinical Names: Ranitidine
Summary
generic name: Ranitidine
trade names: Zantac®, Zantac 75®
type of drug: H2-receptor antagonists (histamine blocker), prevents the secretion of acid into the stomach.
used to treat: Gastroesophageal reflux disease, erosive esophagitis, heartburn, stomach and duodenal ulcers, and Zollinger-Ellison syndrome.
overview of interactions:
• nutrient affected by drug: Iron
• nutrient affecting drug performance: Magnesium
• nutrient affected by drug: Zinc
• nutrient affected by drug: Vitamin B12
(Cyanocobalamin)
• substance toxicity affected by drug: Alcohol
• herb affecting drug performance: Nicotiana species (Tobacco)
Interactions
nutrient affected by drug: Iron
• mechanism: Gastric acid secretion has been demonstrated to facilitate iron absorption. Malabsorption of dietary iron appears to result from inhibition of gastric secretion by the H2-receptor antagonists. H2-receptor antagonists. are also considered efficient chelators of Fe2+. While this probably lacks clinical importance during the short term, long term use of ranitidine and related drugs could contribute to the occurrence of iron-deficiency anemia. Such impairment of nonheme iron absorption could be amplified for individuals also using antacids on a regular basis along with the ranitidine.
(Aymard JP, et al. Med Toxicol Adverse Drug Exp 1988 Nov-Dec;3(6):430-448.)
• nutritional support: Anemia due to blood loss is common among the users of ranitidine because many suffer from ulcers. Before starting to supplement with iron individuals taking ranitidine should discuss their concern about potential for iron depletion with their prescribing physician and/or a nutritionally trained healthcare professional. Iron levels can be tested through standard laboratory tests. In the event that iron supplementation is determined to be necessary, a typical adult dose is 100 mg per day.
nutrient affecting drug performance: Magnesium
• research: The concern regarding potential interaction between ranitidine and magnesium does not primarily originate with a drug-nutritient interaction per se. Instead, research on the interaction between magnesium hydroxide and ranitidine has found that some antacids reduce the bioavailability of the H2-receptor antagonists. This is particularly true when ranitidine is used at the same time as high doses of the relevant antacids. Bachmann et al found that among healthy subjects, i.e., ones who would not normally use the drug, a magnesium hydroxide/aluminum hydroxide antacid decreased ranitidine absorption by 20%-25% when the two substances were taken at the same time.
(Bachmann KA. et al. Scand J Gastroenterol Suppl 1994;206:14-19; Propst A, et al.
Arzneimittelforschung 1996 Jun;46(6):621-624.)
• nutritional support: In relation to antacid use, the potential for interaction can be reduced by taking the ranitidine at least two hours before or after any antacid containing aluminum or magnesium. There is concern that a multivitamin/mineral supplement containing magnesium could have the same effect, especially if the magnesium is in the form of magnesium hydroxide. In such cases, caution would advise taking the ranitidine at least two hours before or after the magnesium-containing supplement.
nutrient affected by drug: Zinc
• research: Reduced gastric acid secretion can exert both direct and indirect effects on the absorption of zinc that parallel it known impairment of iron absorption. In a study involving eleven healthy adults, Sturniolo et al found that 300 mg/day of ranitidine for 3 days significantly reduced gastric acid and zinc absorption.
(Sturniolo GC, et al. J Am Coll Nutr 1991 Aug;10(4):372-375.)
• nutritional support: Normally a moderate daily intake of zinc, 15-25 mg, would be sufficient to prevent deficiencies. However, higher doses, in the range of 34-50 mg three times per day, might be beneficial in situations of impaired zinc absorption. Before starting to supplement with zinc individuals taking ranitidine should discuss their concern about potential for zinc depletion with their prescribing physician and/or a nutritionally trained healthcare professional.
nutrient affected by drug: Vitamin B12
(Cyanocobalamin)
• mechanism: H2-receptor antagonists such as ranitidine decrease acid secretion by the gastric parietal cells. Gastric acid and pepsin produced by these cells are required for the cleavage of vitamin B12 from dietary sources. Thus, ranitidine prevents dietary B12 from being freed from its protein binder during digestion. Further, intrinsic factor (IF), also produced by gastric parietal cells, is required for vitamin B12 absorption from the gastrointestinal tract. Although ranitidine, or other H2-receptor antagonists, have not conclusively been shown to decrease IF secretion, studies have demonstrated a significant reduction in food-bound vitamin B12 absorption secondary to decreased acid secretion in patients taking these drugs. While this probably lacks clinical importance during the short term, long term use of ranitidine and related drugs could contribute to the occurrence of vitamin B12-deficiency anemia. Such impairment of vitamin B12 absorption could be amplified for individuals also using antacids on a regular basis along with the ranitidine.
(Hartshorn, EA. Ann Pharmacother 1992;26:1283-1286; Force RW, Nahata MC. Ann Pharmacother
1992 Oct;26(10):1283-1286; Aymard JP, et al. Med Toxicol Adverse Drug Exp 1988 Nov-Dec;3(6):430-448.)
• nutritional concerns: Patients using ranitidine, or other H2-receptor antagonists, have the potential to suffer from vitamin B12 deficiency. The risk of such deficiency is significantly greater among individuals who have inadequate stores of vitamin B12, most likely as a result of poor diet, before they start using the drug. Furthermore, anemia due to blood loss is common among the users of ranitidine because many suffer from ulcers.
• nutritional support: Normally vitamin B12 supplementation is unnecessary except among vegans. Before starting to supplement with vitamin B12 individuals taking ranitidine should discuss their concern about potential for iron depletion with their prescribing physician and/or a nutritionally trained healthcare professional. Vitamin B12 levels can be tested through standard laboratory tests. In the event that supplementation is determined to be necessary, a typical adult dose of 10-25 mcg per day might be beneficial. It is important to note that the form of vitamin B12 typically found in supplements does not require stomach acid to be absorbed.
substance toxicity affected by drug: Alcohol
• research: Ranitidine reduces alcohol breakdown causing serum alcohol levels to increase substantially. Studies with rats have indicate that ranitidine, and to some degree other H2-receptor antagonists, impair alcohol dehydrogenase (ADH) activity when taken at therapeutic doses. Ranitidine also demonstrated either mixed or competitive inhibition of rat hepatic ADH. Thus, in alcoholics and in social drinkers who require treatment with H2-receptor antagonists, the prescribing physicians might choose to prescribe famotidine, instead of ranitidine or cimitidine, as a less problematic H2 blocker. Of course, stopping alcohol consumption represents a more direct route to eliminating this risk and would most likely also provide significant benefits in reducing the symptoms for which the ranitidine was originally prescribed. Even so, the levels of alcohol intake necessary to induce this interactions may be significantly higher than commonly consumed by most social drinkers on any consistent basis.
(Caballeria J, et al. Dig Dis Sci 1991 Dec;36(12):1673-1679.)
herb affecting drug performance: Nicotiana species
(Tobacco)
• research: Tobacco smoking has been shown to impair the therapeutic effect of H2-receptor antagonists. In their study involving eighteen healthy volunteers, half of whom smoked, Schurer-Maly et al found that smoking decreased the acid blocking effects of ranitidine.
(Schurer-Maly et al. Scand J Gastroenterol 1989 Dec;24(10):1172-1178; Bauerfeind P, et al.
Gut 1987 May;28(5):549-556.)
Please read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
Aymard JP, Aymard B, Netter P, Bannwarth B, Trechot P, Streiff F. Haematological adverse effects of histamine H2-receptor antagonists.
Med Toxicol Adverse Drug Exp 1988 Nov-Dec;3(6):430-448.
Abstract: Histamine H2-receptor antagonists are widely used in the treatment of gastrointestinal diseases related to gastric acid hypersecretion. Cimetidine was introduced into medical practice in 1976 and ranitidine, famotidine and nizatidine in 1981, 1985 and 1987, respectively. Haematological adverse effects are relatively uncommon and most have been reported in cases of cimetidine administration. These adverse effects are reviewed under 4 main headings: (a) blood cytopenias and leucocytosis; (b) coagulation disorders related to drug interactions with oral anticoagulants; (c) reduction of dietary iron absorption; and (d) reduction of dietary cobalamin absorption. 85 reported cases of blood cytopenias attributed to these drugs are reviewed, of which 75 (88%) were associated with cimetidine therapy. In postmarketing surveillance studies, the incidence of cimetidine-associated blood cytopenia has been evaluated at about 2.3 per 100,000 patients. Neutropenia and agranulocytosis are by far the most frequently encountered. Whatever the drug or the type of cytopenia, this adverse effect is almost always rapidly reversible when treatment is stopped. Moreover, in several cases other factors such as underlying diseases or additional drugs could have been responsible, at least partly, for the cytopenia. The pathophysiological basis of these adverse effects remains poorly explained. Various mechanisms have been proposed, which in some cases are probably associated: (a) direct toxicity for haemopoietic stem cells; (b) drug-induced immune reactions leading to blood or bone marrow cell damage, and (c) drug interactions, with increased and prolonged action of potentially haematotoxic drugs. Mechanisms (a) and (c) appear to be of particular clinical importance in cases of impaired renal elimination of H2-receptor antagonists. Cimetidine and probably to a lesser extent ranitidine potentiate the action of oral anticoagulants of both coumarin and indanedione structure. This may result in haemorrhagic complications. Such action is a consequence of the reduced hepatic metabolism of oral anticoagulants through a dose-dependent, reversible inhibition of cytochrome P450. Malabsorption of dietary iron and cobalamin appears to result from inhibition of gastric secretion by the H2-receptor antagonists. This is of no clinical importance in short term treatment, but long term use of H2-receptor antagonists may theoretically contribute to the occurrence of iron or cobalamin deficiency anaemia.
Bachmann KA, Sullivan TJ, Jauregui L, Reese J, Miller K, Levine LDrug interactions of H2-receptor antagonists.
Scand J Gastroenterol Suppl 1994;206:14-19.
Abstract: Three drug interactions of nizatidine and of other antisecretory agents were studied comparatively. First, the effects of nizatidine, cimetidine and ranitidine on the dispositional kinetics of theophylline were evaluated in chronic obstructive pulmonary disease (COPD) patients. Second, the effect of magnesium/aluminium hydroxide on the relative bioavailability of nizatidine, famotidine, cimetidine and ranitidine was evaluated in healthy volunteers. Finally, the effects of nizatidine and omeprazole on the dispositional kinetics of phenytoin were evaluated in healthy volunteers. Only cimetidine altered the steady-state kinetics of oral theophylline, slowing theophylline clearance by 25%. Each of the H2-receptor antagonists exhibited a modest decline in relative bioavailability when ingested with antacid. Antacid ingestion decreased the bioavailability of famotidine, ranitidine and cimetidine by 20-25%, and the bioavailability of nizatidine by 12%. Each of these effects was statistically significant. Finally, it was found that neither omeprazole nor nizatidine affected the single dose kinetics of phenytoin.
Bauerfeind P, Cilluffo T, Fimmel CJ, Emde C, von Ritter C, Kohler W, Gugler R, Gasser T, Blum AL. Does smoking interfere with the effect of histamine H2-receptor antagonists on intragastric acidity in man?
Gut 1987 May;28(5):549-556.
Caballeria J, Baraona E, Deulofeu R, Hernandez-Munoz R, Rodes J, Lieber CS. Effects of H2-receptor antagonists on gastric alcohol dehydrogenase activity.
Dig Dis Sci 1991 Dec;36(12):1673-1679.
Abstract: Inhibition of gastric alcohol dehydrogenase (ADH) activity by cimetidine results in elevated blood levels of ethanol after moderate consumption. To search for alternative H2-blockers lacking such an effect, we compared cimetidine, ranitidine, nizatidine, and famotidine. They inhibited rat gastric ADH noncompetitively, with a Ki for ethanol oxidation of 0.68 mM for cimetidine, 0.5 mM for ranitidine, 1 mM for nizatidine, and 4.5 mM for famotidine. These concentrations are higher than therapeutic plasma levels, but intracellular concentrations in the gastric mucosa (assessed with [3H]cimetidine and [14C]famotidine) were at least 10- and 2-fold greater than in the blood, respectively. These results suggests that, given at therapeutic doses in vivo, the degree of inhibition by cimetidine and ranitidine should be significant and comparable, that by nizatidine should be smaller, and that by famotidine should be negligible. These drugs also exerted either mixed or competitive inhibition of rat hepatic ADH, but the effects of cimetidine and famotidine were observed at concentrations unlikely to occur in vivo. Thus, in alcoholics and in social drinkers who require treatment with H2-receptor antagonists, famotidine might be preferable to the other H2 blockers tested.
Desager JP, Harvengt C. Oral bioavailability of nizatidine and ranitidine concurrently administered with antacid.
J Int Med Res 1989 Jan-Feb;17(1):62-67.
Desmond PV, Harman PJ, Gannoulis N, Kamm M, Mashford ML. The effect of an antacid and food on the absorption of cimetidine and ranitidine.
J Pharm Pharmacol 1990 May;42(5):352-354.
Abstract: The effect of varying doses of a liquid antacid preparation containing magnesium hydroxide, aluminum hydroxide and simethicone on the absorption of the H2-receptor antagonists, cimetidine and ranitidine, was determined in 2 groups of 11 volunteers; one group fasted and one group fed a standardized breakfast. The antacid alone caused a significant decrease in the AUC of cimetidine (24%). Similarly, concomitant antacid caused a 59% decrease in the AUC of ranitidine. There were no effects on any of the other pharmacokinetic parameters examined. The absorption of both drugs was similar in fasted and fed volunteers, but in the fed volunteers the antacid did not produce the decrease in AUC seen in the fasted volunteers. These data suggest that H2-receptor antagonists should not be taken at the same time as antacids.
Force RW, Nahata MC. Effect of histamine H2-receptor antagonists on vitamin B12 absorption.
Ann Pharmacother 1992 Oct;26(10):1283-1286.
Abstract: OBJECTIVE: To discuss the potential of histamine H2-receptor antagonists (H2RAs) to cause malabsorption of vitamin B12 (cyanocobalamin). DATA SOURCES: Pertinent literature was identified via a MEDLINE search. Journals and references cited in published articles also were used as data sources. STUDY SELECTION: Studies evaluating the effect of H2RAs on vitamin B12 absorption were reviewed. DATA SYNTHESIS: H2RAs decrease acid secretion by the gastric parietal cells. Gastric acid and pepsin produced by these cells are required for the cleavage of vitamin B12 from dietary sources. Intrinsic factor (IF), also produced by gastric parietal cells, is required for vitamin B12 absorption from the gastrointestinal tract. Although H2RAs have not conclusively been shown to decrease IF secretion, studies have demonstrated a significant reduction in food-bound vitamin B12 absorption secondary to decreased acid secretion in patients taking these drugs. CONCLUSIONS: H2RAs have the potential to cause vitamin B12 deficiency. This may be important in patients with inadequate stores of vitamin B12 (e.g., poor diet), particularly those receiving H2RA therapy continuously for more than two years. Healthcare providers should be aware of this potential adverse effect.
Gugler R, Allgayer H. Effects of antacids on the clinical pharmacokinetics of drugs. An update.
Clin Pharmacokinet 1990 Mar;18(3):210-219. (Review)
Hartshorn, EA. Effect of Histamine H2-Receptor Antagonists on Vitamin B12 Absorption.
Ann Pharmacother 1992;26:1283-1286.
Lapenna D, De Gioia S, Mezzetti A, Grossi L, Festi D, Marzio L, Cuccurullo F. H2-receptor antagonists are scavengers of oxygen radicals. Eur J Clin Invest 1994 Jul;24(7):476-481.
Abstract: Potential oxygen radical scavenging properties of the H2-receptor antagonists cimetidine, ranitidine and famotidine were investigated. These drugs, although ineffective against superoxide anion and hydrogen peroxide, can scavenge hydroxyl radical (OH.) with a very high rate constant, which is about tenfold higher than that of the specific scavenger mannitol for famotidine (1.7 x 10(10) mol-1 s-1) and cimetidine (1.6 x 10(10) mol-1 s-1), ranitidine displaying a rate constant of 7.5 x 10(9) mol-1 s-1. These OH. savenging effects are significant beginning from 10, 28 and 100 mumol l-1 concentration for famotidine, cimetidine and ranitidine, respectively, thus suggesting that the drugs may effectively act as OH. scavengers in vivo especially in the gastric lumen. Only cimetidine can apparently bind and inactivate iron, which further emphasizes its antioxidant capacity. Moreover, all drugs, even at 10 mumol l-1 concentration, show powerful scavenging effects on hypochlorous acid and monochloramine, which are cytotoxic oxidants arising from inflammatory cells, such as neutrophils. These data suggest that some therapeutical effects of H2-receptor antagonists in peptic ulcer may also be related to their antiradical-antioxidant capacity, and that these drugs could potentially be used in other disease entities characterized by free radical-mediated oxidative stress in vivo.
Propst A, Propst T, Judmaier G. Comparison of the effects of ranitidine effervescent tablets and magnesium hydroxide-aluminium oxide on intragastric acidity. A single-centre, randomised, open cross-over study.
Arzneimittelforschung 1996 Jun;46(6):621-624.
Abstract: In previous studies measuring intragastric pH in healthy volunteers it was shown that there was a faster onset of action with ranitidine (CAS 66357-35-5) 300 mg effervescent tablets (Zantac) compared to standard tablets. In a single-centre, randomised, open cross-over study the pH-values obtained over 6 h following the administration of one ranitidine 150 mg effervescent tablet were compared with those after aluminium oxide-magnesium hydroxide (algeldrate, CAS 1330-44-5, Al-Mg-hydroxide) 10 ml and placebo in healthy volunteers. 24 healthy male subjects between 19 and 32 years of age entered the study, 19 subjects were available for all three measurements. After an overnight fast, intragastric pH was monitored for 7 h using a glass electrode and a digital data recorder. The time in % during which the pH was > or = 3.5 and the area under the curve of the obtained pH-curves were compared. There was a highly statistically significant difference between ranitidine effervescent tablets versus Al-Mg-hydroxide and placebo whereas there was no such difference between Al-Mg-hydroxide and placebo. The onset of action of ranitidine effervescent tablets was almost immediate. It is concluded that there was a clear superiority of ranitidine effervescent tablets in healthy volunteers and it is suggested that pH-metry in patients with acidity-related diseases should be investigated for a better understanding of the function of effervescent tablets.
Schurer-Maly CC, Varga L, Koelz HR, Halter F. Smoking and pH response to H2-receptor antagonists.
Scand J Gastroenterol 1989 Dec;24(10):1172-1178.
Abstract: Smoking has been shown to impair the therapeutic effect of H2-receptor antagonists. To evaluate the acid-reducing capacity of H2-receptor antagonists in relation to smoking habits, we tested the effect of ranitidine (Ran) and famotidine (Fam) under physiologic conditions, using ambulatory pH-metry. Intragastric pH was measured over 20 h. Each of 18 healthy volunteers, 9 smokers and 9 nonsmokers, received 40 mg Fam, 300 mg Ran, or placebo in a double-blind, randomized study as a single evening dose. With both drugs 20-h acidity was markedly suppressed. After Fam treatment mean inhibition was 61% in smokers and 76% in nonsmokers and after Ran 51% and 67%, respectively. When areas under the pH curves for each individual were calculated and treatment compared with placebo (= 100%), the response was smaller in smokers than in nonsmokers with either drug (Fam, 153 +/- 21% versus 214 +/- 19%, p less than 0.01; Ran, 176 +/- 21% versus 232 +/- 29%, p less than 0.05) during the first 4 h after drug intake. A similar effect was observed in the morning period from 0600 to 1000 h (Fam, 118 +/- 19% versus 206 +/- 19%, p less than 0.001; Ran, 133 +/- 21% versus 207 +/- 31%, p less than 0.02). During the nighttime there were no significant differences. These findings indicate that smoking impairs the response to both drugs tested.
Streeter AM, Goulston KJ, Bathur FA, Hilmer RS, Crane GG, Pheils MT. Cimetidine and malabsorption of cobalamin.
Dig Dis Sci 1982 Jan;27(1):13-16.
Sturniolo GC, Montino MC, Rossetto L, Martin A, D'Inca R, D'Odorico A, Naccarato R. Inhibition of gastric acid secretion reduces zinc absorption in man.
J Am Coll Nutr 1991 Aug;10(4):372-375.
Abstract: Numerous factors seem to affect zinc absorption. Gastric acid secretion has been demonstrated to facilitate iron absorption. The zinc tolerance test (ZTT with ZnSO4 220 mg p.o.) was performed in 11 healthy volunteers to study the effects of administering the acid secretion inhibitor cimetidine (1 g/day p.o. for 3 days) and to evaluate the influence of HCl gastric secretion on zinc absorption in physiological conditions. Zinc absorption was reduced after cimetidine administration (p less than 0.005), suggesting that gastric pH influences zinc absorption. To rule out any direct effect of the drug on zinc absorption in five other healthy adults we further evaluated zinc absorption by using a different H2 antagonist (ranitidine 300 mg/day for 3 days and 300 mg before the test). Cimetidine was also tested in these subjects at half the dosage administered to the first group of subjects. Gastric acidity was monitored at 60-min intervals throughout the test via a nasogastric tube. The areas under the plasma concentration curves for zinc were significantly reduced after ranitidine (p less than 0.01), but not after cimetidine administration. Gastric acid was also reduced after ranitidine, but not after cimetidine (500 mg) administration, suggesting that gastric acid secretion plays a role in the regulation of zinc absorption in man.
Sullivan TJ, Reese JH, Jauregui L, Miller K, Levine L, Bachmann KA. Short report: a comparative study of the interaction between antacid and H2-receptor antagonists.
Aliment Pharmacol Ther 1994 Feb;8(1):123-126.
Teixeira F, Geraldes CF, Gil VM, Helena M, Teixeira SF. In vitro complexation of aluminum and magnesium by cimetidine and ranitidine. A nuclear magnetic resonance study.
Gastroenterol Clin Biol 1984 Nov;8(11):879-80. (Letter)
Threlkeld DS, ed. Gastrointestinal Drugs, Histamine H2 Antagonists. In: Facts and Comparisons Drug Information. St. Louis, MO: Facts and Comparisons, Sep 1995.
van Zyl JM, Kriegler A, van der Walt BJ. Anti-oxidant properties of H2-receptor antagonists. Effects on myeloperoxidase-catalysed reactions and hydroxyl radical generation in a ferrous-hydrogen peroxide system.
Biochem Pharmacol 1993 Jun 22;45(12):2389-2397.