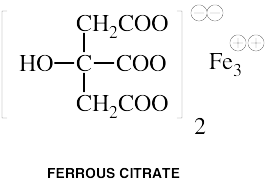

Iron
Common Names: Ferrous citrate, Ferrous gluconate, Ferrous succinate, Ferrous sulfatePlease read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
[No author given.] Infect Dis News 1990(June):17.
Albitar S, Genin R, Fen-Chong M, Serveaux MO, Bourgeon B. High dose enalapril impairs the response to erythropoietin treatment in haemodialysis patients.
Nephrol Dial Transplant 1998 May;13(5):1206-1210.
Abstract: BACKGROUND: The resistance to recombinant human erythropoietin (rHuEpo) therapy in haemodialysis (HD) patients has multifactorial aetiologies: erythropoietin insufficiency, dialysis insufficiency, iron deficiency, and secondary hyperparathyroidism. Angiotensin-converting enzyme (ACE) inhibitors induce anaemia in patients with essential hypertension, congestive heart failure, chronic renal insufficiency, and renal transplants. Data exist suggesting that ACE inhibitors impair erythropoiesis in HD patients. Therefore the aim of this study was to investigate the impact of enalapril on rHuEpo requirement. METHODS: In the present prospective non-randomized study of 12 months, we compared the effects of enalapril and nifedipine on rHuEpo requirement in 40 hypertensive patients receiving rHuEpo for more than 6 months on maintenance haemodialysis. Twenty normotensive rHuEpo-dependent patients served as a control group. All patients with severe hyperparathyroidism or iron deficiency were excluded. RESULTS: The mean (+/- SD) haemoglobin concentration was > 10 g/dl in all groups. The mean weekly rHuEpo dose increased in the enalapril group (P<0.0001 vs before) and remained constant in the nifedipine and control groups (P=NS vs before). Statistically, there was no differences with regard to iPTH levels, dialysis parameters, iron status, and underlying renal diseases among all groups. CONCLUSION: High-dose enalapril increases rHuEpo requirement and should be reserved for dialysis patients with hypertension uncontrollable with other antihypertensive medications or dialysis patients with cardiac failure.
Aymard JP, Aymard B, Netter P, Bannwarth B, Trechot P, Streiff F. Haematological adverse effects of histamine H2-receptor antagonists.
Med Toxicol Adverse Drug Exp 1988 Nov-Dec;3(6):430-448.
Abstract: Histamine H2-receptor antagonists are widely used in the treatment of gastrointestinal diseases related to gastric acid hypersecretion. Cimetidine was introduced into medical practice in 1976 and ranitidine, famotidine and nizatidine in 1981, 1985 and 1987, respectively. Haematological adverse effects are relatively uncommon and most have been reported in cases of cimetidine administration. These adverse effects are reviewed under 4 main headings: (a) blood cytopenias and leucocytosis; (b) coagulation disorders related to drug interactions with oral anticoagulants; (c) reduction of dietary iron absorption; and (d) reduction of dietary cobalamin absorption. 85 reported cases of blood cytopenias attributed to these drugs are reviewed, of which 75 (88%) were associated with cimetidine therapy. In postmarketing surveillance studies, the incidence of cimetidine-associated blood cytopenia has been evaluated at about 2.3 per 100,000 patients. Neutropenia and agranulocytosis are by far the most frequently encountered. Whatever the drug or the type of cytopenia, this adverse effect is almost always rapidly reversible when treatment is stopped. Moreover, in several cases other factors such as underlying diseases or additional drugs could have been responsible, at least partly, for the cytopenia. The pathophysiological basis of these adverse effects remains poorly explained. Various mechanisms have been proposed, which in some cases are probably associated: (a) direct toxicity for haemopoietic stem cells; (b) drug-induced immune reactions leading to blood or bone marrow cell damage, and (c) drug interactions, with increased and prolonged action of potentially haematotoxic drugs. Mechanisms (a) and (c) appear to be of particular clinical importance in cases of impaired renal elimination of H2-receptor antagonists. Cimetidine and probably to a lesser extent ranitidine potentiate the action of oral anticoagulants of both coumarin and indanedione structure. This may result in haemorrhagic complications. Such action is a consequence of the reduced hepatic metabolism of oral anticoagulants through a dose-dependent, reversible inhibition of cytochrome P450. Malabsorption of dietary iron and cobalamin appears to result from inhibition of gastric secretion by the H2-receptor antagonists. This is of no clinical importance in short term treatment, but long term use of H2-receptor antagonists may theoretically contribute to the occurrence of iron or cobalamin deficiency anaemia.
Bays HE, Dujovne CA. Drug interactions of lipid-altering drugs. Drug Saf. 1998 Nov;19(5):355-371. (Review)
Beard J, Borel M, Peterson FJ. Changes in iron status during weight loss with very-low-energy diets.
Am J Clin Nutr 1997 Jul;66(1):104-110.
Abstract: For several decades, very-low-energy diets (VLEDs) have been used by obese individuals to achieve weight loss. During the weight loss, patients often have dramatic drops in circulating thyroid hormone concentrations and experience cold intolerance. Because poor iron status is known to alter thermogenesis, we investigated the possibility that iron intake interacts with energy intake during weight loss in obese individuals. The effects on indicators of iron and thyroid status of increasing the iron content of a VLED from 18 to 27 mg/d during 12 wk of a VLED were compared with the effects on the same indicators of increasing energy intake from 1752 kJ(420 kcal) to 3347 kJ(800 kcal)/d. Although all VLED groups initially had 30% declines in plasma transferrin saturation, increases in plasma ferritin concentrations, and decreases in plasma thyroid hormone concentrations, patients who received iron supplementation had significantly higher circulating concentrations of triiodothyronine and thyroxine at the end of the VLED than did patients who received only the recommended dietary allowance of iron. The patients who received iron supplementation also had a more rapid return of iron indicators to normal values over the course of the VLED. The transitory fall in iron delivery to bone marrow was not associated with anemia. These data suggest that higher thyroid hormone concentrations can be maintained during VLEDs that provide higher iron intakes.
Beard JL, Borel MJ, Derr J. Impaired thermoregulation and thyroid function in iron-deficiency anemia.
Am J Clin Nutr 1990 Nov;52(5):813-819.
Abstract: Ten women with iron-deficiency anemia, 8 with depleted iron stores (nonanemic), and 12 control women, all of similar body fatness, were exposed to a 28 degrees C water bath to test the hypothesis that iron-deficiency anemia impairs thermoregulatory performance. The anemic women had lower rectal temperatures than did control women (36.0 +/- 0.2 vs 36.2 +/- 0.1 degree C, respectively, P = 0.001) and a lower rate of oxygen consumption (5.28 +/- 0.26 vs 5.99 +/- 0.29 mL.min-1.kg body wt-1, respectively, P = 0.04) at 100 min of cold exposure. Plasma thyroxine and triiodothyronine concentrations were significantly (P less than 0.002) lower in anemic than in control women at baseline and during cold exposure. Responses of iron-depleted subjects were similar to those of control subjects. Iron supplementation corrected the anemia, significantly (P = 0.03) improved rectal temperature at 100 min, and partially normalized plasma thyroid hormone concentrations. Plasma catecholamines were unaffected by iron status. This experiment demonstrates a functional consequence of iron-deficiency anemia in the balance of heat production and loss and suggests that thyroid-hormone metabolism may be responsible.
Beard JL, Brigham DE, Kelley SK, Green MH. Plasma thyroid hormone kinetics are altered in iron-deficient rats.
J Nutr 1998 Aug;128(8):1401-1408.
Abstract: Iron deficiency anemia is associated with lower plasma thyroid hormone concentrations in rodents and, in some studies, in humans. The objective of this project was to determine if plasma triiodothyronine (T3) and thyroxine (T4) kinetics were affected by iron deficiency. Studies were done at a near-thermoneutral temperature (30 degrees C), and a cool environmental temperature (15 degrees C), to determine plasma T3 and T4 kinetics as a function of dietary iron intake and environmental need for the hormones. Weanling male Sprague-Dawley rats were fed either a low Fe diet [iron-deficient group (ID), <5 microg/g Fe] or a control diet [control group (CN), 35 microg/g Fe] at each temperature for 7 wk before the tracer kinetic studies. An additional ID group receiving exogenous thyroid hormone replacement was also used at the cooler temperature. For T4, the disposal rate was >60% lower (89 +/- 6 vs. 256 +/- 53 pmol/h, P < 0.001) in ID rats than in controls at 30 degrees C, and approximately 40% lower (192 +/- 27 vs. 372 +/- 26 pmol/h, P < 0.01) in ID rats at 15 degrees C. Exogenous T4 replacement in a cohort of ID rats at 15 degrees C normalized the T4 concentration and the disposal rate. For T3, the disposal rate was significantly lower in ID rats in a cool environment (92 +/- 11 vs. 129 +/- 11 pmol/h, P < 0.01); thyroxine replacement again normalized the T3 disposal rate (126 +/- 12 pmol/h). Neither liver nor brown fat thyroxine 5'-deiodinase activities were sufficiently different to explain the lower T3 disposal rates in iron deficiency. Thus, plasma thyroid hormone kinetics in iron deficiency anemia are corrected by simply providing more thyroxine. This suggests a central regulatory defect as the primary lesion and not peripheral alterations.
Ben-Shachar D, Youdim MB. Neuroleptic-induced supersensitivity and brain iron: I. Iron deficiency and neuroleptic-induced dopamine D2 receptor supersensitivity.
J Neurochem 1990 Apr;54(4):1136-1141.
Abstract: Previous studies have shown that nutritional iron deficiency in rats reduces brain iron content, resulting in dopamine D2 receptor subsensitivity, as indicated by a decrease in [3H]spiperone binding in caudate nucleus and in behavioral responses to apomorphine. Both phenomena can be reversed by iron supplementation. The possibility that neuroleptic-induced dopamine D2 receptor supersensitivity involves an alteration in brain iron content was investigated in nutritionally iron-deficient and control rats chronically treated with haloperidol (5 mg/kg daily for 14 or 21 days). Neuroleptic treatment was initiated either (a) concurrently with iron deficiency or (b) 2 weeks after the start of iron deficiency. The results show that dopamine D2 receptor subsensitivity, a feature of iron deficiency, is absent in haloperidol-treated, iron-deficient groups. On the contrary, these animals demonstrated biochemical and behavioral dopamine D2 receptor supersensitivity that is relatively greater than that observed with control, haloperidol-treated animals. Haloperidol (5 mg/kg daily for 21 days) as well as chlorpromazine (10 mg/kg daily for 21 days) caused a significant reduction (20-25%) in liver nonheme iron stores as compared with values in control rats. However, in iron-deficient rats, in which liver iron stores were almost totally depleted, haloperidol had no effect. The ability of chronic haloperidol treatment to prevent the reduction of dopamine D2 receptor number during iron deficiency may be associated with alteration of body iron status. Thus, less iron may result in an increase in free haloperidol available to the dopamine D2 receptor.
Brouwers JR. Drug interactions with quinolone antibacterials. Drug Saf 1992 Jul-Aug;7(4):268-281. (Review)
Abstract: The quinolone antibacterials are prone to many interactions with other drugs. Quinolone absorption is markedly reduced with antacids containing aluminium, magnesium and/or calcium and therapeutic failure may result. Other metallic ion-containing drugs, such as sucralfate, iron salts, and zinc salts, can also reduce absorption. Some of the newer quinolones inhibit the cytochrome P450 system, e.g. enoxacin, pefloxacin and ciprofloxacin. The toxicity of drugs that are metabolised by the cytochrome P450 system is enhanced by concomitant use of some quinolones. Ciprofloxacin, enoxacin and pefloxacin can increase theophylline concentrations to toxic values. The pharmacokinetics of warfarin and cyclosporin are unaffected. Ofloxacin, fleroxacin and temafloxacin have a low inhibitory effect on the cytochrome P450 system and a low interaction potential may result. The affinity of quinolones for the gamma-aminobutyric acid (GABA) receptor may induce CNS adverse effects; these effects are enhanced by some nonsteroidal anti-inflammatory drugs (NSAIDs).
Campbell N, Paddock V, Sundaram R. Alteration of methyldopa absorption, metabolism, and blood pressure control caused by ferrous sulfate and ferrous gluconate.
Clin Pharmacol Ther 1988 Apr;43(4):381-386.
Abstract: This study examined the effect of two widely used iron treatments on methyldopa absorption, metabolism, and blood pressure control. A 500 mg tablet of methyldopa (2.37 mmol) was taken with and without ferrous sulfate (325 mg) by 12 normal subjects in a randomized crossover trial. When ferrous sulfate was taken with methyldopa there was a decrease in the proportion of methyldopa excreted as "free" methyldopa (49.5% +/- 12.4% vs 21.1% +/- 4.77%; p less than 0.01), a significant increase in the proportion excreted as methyldopa sulfate (37.8% +/- 12.3% vs 65.8% +/- 10.5%; p less than 0.01), and a decrease in the percentage of methyldopa absorbed (29.1% +/- 12.5% vs 7.88% +/- 4.14%; p less than 0.01). These factors resulted in an 88% reduction in the quantity of "free" methyldopa excreted. To determine if an iron preparation without sulfate produced the same effect, the study was repeated with ferrous gluconate (600 mg) with similar results. The clinical consequences of the methyldopa-ferrous sulfate interaction was determined in five hypertensive subjects receiving chronic methyldopa therapy. The subjects took ferrous sulfate for 2 weeks. There was an increase in both systolic and diastolic blood pressure in four patients and a decrease in blood pressure in all patients after ferrous sulfate was discontinued. The increases in blood pressure were substantial in three of the patients.
Campbell NR, Campbell RR, Hasinoff BB. Ferrous sulfate reduces methyldopa absorption: methyldopa: iron complex formation as a likely mechanism.
Clin Invest Med 1990 Dec;13(6):329-332.
Abstract: Ferrous sulfate and sodium sulfate reduce methyldopa absorption in humans. This current study was conducted to investigate some of the potential factors by which these compounds could reduce methyldopa absorption. A rat model developed to examine drug absorption was used. Solutions of 14C methyldopa alone and with ferrous sulfate or sodium sulfate were injected in vivo into closed duodenal segments. Ferrous sulfate reduced methyldopa absorption 52.9% (p less than 0.01), while sodium sulfate had no significant effect on methyldopa absorption. In vitro iron in its ferrous form rapidly oxidizes to the ferric form in the presence of methyldopa. The ferric form of iron binds strongly to methyldopa, presumably resulting in the decreased methyldopa absorption. Methyldopa was stable in vivo and in vitro in the presence of ferrous sulfate and sodium sulfate. These studies are consistent with ferrous sulfate reducing methyldopa absorption by the formation of ferric iron: methyldopa complexes.
Campbell NR, Hasinoff BB, Stalts H, Rao B, Wong NC. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism.
Ann Intern Med 1992 Dec 15;117(12):1010-1013.
Abstract: OBJECTIVE: To determine whether simultaneous ingestion of ferrous sulfate and thyroxine reduces the efficacy of thyroid hormone in patients with primary hypothyroidism. DESIGN: Uncontrolled clinical trial. SETTING: Outpatient research clinic of a tertiary care center. PATIENTS: Fourteen patients with established primary hypothyroidism on stable thyroxine replacement. INTERVENTION: All patients were instructed to ingest simultaneously, a 300-mg ferrous sulfate tablet and their usual thyroxine dose every day for 12 weeks. RESULTS: After 12 weeks of ferrous sulfate ingestion with thyroxine, the mean level of serum thyrotropin (thyroid stimulating hormone, TSH) rose from 1.6 +/- 0.4 to 5.4 +/- 2.8 mU/L (P < 0.01), but the free thyroxine index did not change significantly. Subjective evaluation using a clinical score showed that nine patients had an increase in symptoms and signs of hypothyroidism; the mean score for the 14 patients changed from 0 to 1.3 +/- 0.4 (P = 0.011). When iron and thyroxine were mixed together in vitro, a poorly soluble purple complex appeared that indicated the binding of iron to thyroxine. CONCLUSIONS: Simultaneous ingestion of ferrous sulfate and thyroxine causes a variable reduction in thyroxine efficacy that is clinically significant in some patients. The interaction is probably caused by the binding of iron to thyroxine.
Campbell NR, Hasinoff BB. Iron supplements: A common cause of drug interactions.
Brit J Clin Pharmacol 1991 Mar;31(3):251-255. (Review)
Abstract: Iron-drug interactions of clinical significance may occur in many patients and involve a large number of therapies. Concurrent ingestion of iron causes marked decreases in the bioavailability of a number of drugs. The affected drugs, tetracycline, tetracycline derivatives (doxycycline, methacycline and oxytetracycline), penicillamine, methyldopa, levodopa, carbidopa and ciprofloxacin have diverse chemical structures and clinical effects. The major mechanism of these drug interactions is the formation of iron-drug complexes (chelation or binding of iron by the involved drug). A large number of other important and commonly used drugs such as thyroxine, captopril and folic acid have been demonstrated to form stable complexes with iron. There is little known about the effects of concurrent therapy with iron supplements for most of the drugs.
Campbell NR, Rankine D, Goodridge AE, Hasinoff BB, Kara M. Sinemet-ferrous sulphate interaction in patients with Parkinson's disease.
Br J Clin Pharmacol 1990 Oct;30(4):599-605.
Abstract: 1. This study examined the effects of administering ferrous sulphate 325 mg with Sinemet (100/25 tablet) on levodopa and carbidopa bioavailability and on signs of Parkinson's disease in nine patients. 2. Ferrous sulphate ingestion with Sinemet resulted in a decrease in levodopa area under the curve (AUC) of 30% (P less than 0.01) and a greater than 75% decrease in carbidopa AUC. Despite a strong relationship between reductions in levodopa AUC and reductions in Sinemet efficacy (r = 0.83, P less than 0.01), the average reduction in Sinemet's efficacy associated with ferrous sulphate did not achieve statistical significance (P = 0.055). 3. Chemical studies indicate that iron forms chemical complexes with carbidopa in a similar manner to levodopa and is a likely mechanism for the drug interactions. 4. AUC when a Sinemet tablet is taken concurrently with a ferrous sulphate tablet appears to be clinically significant in some but not all patients. The clinical significance of repeated ingestion of ferrous sulphate with Sinemet requires further studies.
Chin TF, Lach JL. Drug diffusion and bioavailability: tetracycline metallic chelation.
Am J Hosp Pharm 1975 Jun;32(6):625-629
Cutler P. Deferoxamine therapy in high-ferritin diabetes. Diabetes 1989;38:1207-1210.
Dabbagh AJ, Trenam CW, Morris CJ, Blake DR. Iron in joint inflammation. Ann Rheum Dis 1993;52:67-73.
Danesh J, Appleby P. Coronary heart disease and iron status. Meta-analyses of prospective studies.
Circulation 1999;99:852-854.
Daoud AS, Batieha A, al-Sheyyab M, Abuekteish F, Hijazi S. Effectiveness of iron therapy on breath-holding spells.
J Pediatr 1997 Apr;130(4):547-550.
Abstract: OBJECTIVE: The objective of this study is to investigate the effect of iron therapy on breath-holding spells (BHS). METHODOLOGY: Sixty-seven children with BHS were enrolled in a clinical trial to evaluate the effect of iron therapy on BHS. At the beginning of therapy, the clinical, laboratory, and demographic characteristics of the patients in the treatment group (n = 33) and placebo group (n = 34) were comparable. Patients were assessed weekly for the first 8 weeks and then every 2 weeks for the next 8 weeks. Response to therapy was assessed by the change in the frequency of BHS. RESULTS: Children treated with iron showed significant reduction in the frequency of BHS (88%) compared with the frequency (6%) in the placebo group. As expected, the treated group showed a significant improvement of a number of blood indexes compared with the placebo group. Baseline mean levels of hemoglobin and total iron binding capacity were predictive of a favorable response to iron treatment. CONCLUSION: Results of this study indicate that iron therapy is effective in the treatment of BHS and that iron-deficient children seem to be more likely to benefit from such therapy. Response to iron therapy was strongly correlated with improvement in blood indexes.
de Valk B, Marx JJ. Iron, atherosclerosis, and ischemic heart disease. Arch Intern Med. 1999 Jul 26;159(14):1542-1548. (Review)
Dukes GE Jr, Duncan BS. Inflammatory bowel disease. In Applied Therapeutics: The Clinical Use of Drugs, 6th ed. Vancouver, WA: Applied Therapeutics, 1995, 24-27.
Farmer JA, Gotto AM Jr. Antihyperlipidaemic agents. Drug interactions of clinical significance.
Drug Saf 1994 Nov;11(5):301-309.
Abstract: The available antihyperlipidaemic drugs are generally safe and effective, and major systemic adverse effects are uncommon. However, because of their complex mechanisms of action, careful monitoring is required to identify and correct potential drug interactions. Bile acid sequestrants are the most difficult of these agents to administer concomitantly, because their nonspecific binding results in decreased bioavailability of a number of other drugs, including thiazide diuretics, digitalis preparations, beta-blockers, coumarin anticoagulants, thyroid hormones, fibric acid derivatives and certain oral antihyperglycaemia agents. Although the incidence is low, nicotinic acid may cause hepatic necrosis and so should not be used with drugs that adversely affect hepatic structure or function. With the HMG-CoA reductase inhibitors, relatively new agents for which clinical data are still being accumulated, the major problems appears to be rhabdomyolysis, associated with the concomitant use of cyclosporin, fibric acid derivatives or erythromycin, and mild, intermittent hepatic abnormalities that may be potentiated by other hepatotoxic drugs. Fibrates also have the potential to cause rhabdomyolysis, although generally only in combination with HMG-CoA reductase inhibitors, and are subject to binding by concomitantly administered bile acid sequestrants. The major interaction involving probucol is a possible additive effect with drugs or clinical conditions that alter the prolongation of the QTc interval, increasing the potential for polymorphic ventricular tachycardia.
Farmer JA, Gotto AM Jr. Choosing the right lipid-regulating agent. A guide to selection.
Drugs 1996 Nov;52(5):649-661.
Frassinelli-Gunderson EP, Margen S, Brown JR. Iron stores in users of oral contraceptive agents.
Am J Clin Nutr 1985 Apr;41(4):703-712.
Abstract: A comparison of serum ferritin and other parameters of iron status was made between 46 women taking oral contraceptive agents (OCAs) for two or more years continuously and 71 women who never took OCAs. The mean serum ferritin level for the OCA users was 39.5 +/- 21.5 ng/ml and the control group mean level was 25.4 +/- 15.96 ng/ml, which is significantly different at p less than 0.001. Serum transferrin, serum iron, TIBC, MCH and MCHC levels were significantly greater for the OCA users group. Significantly lower RBC and hematocrit levels were found for OCA users while other parameters, hemoglobin, MCV and percent transferrin saturation, were not significantly different. No major differences in subject characteristics and dietary traits were evidenced, except a difference in reported menstrual cycle losses and a higher heme iron content in the diet of the OCA users.
Ganchev T, Negrev N, Mileva V. Effects of indomethacin on erythropoiesis and plasma iron in rats.
Acta Physiol Pharmacol Bulg 1989;15(2):53-57.
Abstract: The effect of indomethacin on erythropoiesis and plasma iron in rats is investigated after 3-day treatment. A more considerable decrease of erythrocytes, haemoglobin and plasma iron is observed, as well as an increase in reticulocytes and 59Fe incorporated in the newly-formed erythrocytes. The plasma bilirubin and lactate levels are also higher. Obviously, this suggests a regeneratory anaemia. It is concluded that prostaglandins are necessary for the normal iron metabolism and for erythropoiesis, but their absence does not prevent entirely the process of erythroid regeneration.
Gossmann J, Thurmann P, Bachmann T, Weller S, Kachel HG, Schoeppe W, Scheuermann EH. Mechanism of angiotensin converting enzyme inhibitor-related anemia in renal transplant recipients.
Kidney Int. 1996 Sep;50(3):973-978.
Greene RJ, Hall AD, Hider RC. The interaction of orally administered iron with levodopa and methyldopa therapy.
J Pharm Pharmacol 1990 Jul;42(7):502-504.
Abstract: The ability of methyldopa and levodopa to interact with both ferrous and ferric iron under a variety of conditions likely to be encountered physiologically has been examined. Spectrophotometric studies of ferrous sulphate in the presence of methyldopa indicate that no complexation occurs below pH2, whilst between pH 4-9, a variety of iron-methyldopa complexes is formed. The formation of these complexes is fast at high pH (pH 9: t1/2 less than 5 s), whilst the rate slows as the pH is lowered (pH 4: t1/2 greater than 30 min). These complexes are characteristic of iron-catecholate species, indicating that in the presence of methyldopa (and levodopa) ferrous iron undergoes autoxidation to the ferric form. The tight binding of ferric iron to methyldopa is predicted to alter the biodistribution characteristics of the complex with respect to the unchelated components. Furthermore, under the acid conditions of the stomach, redox cycling can occur. This will result in both catechol oxidation and production of the toxic hydroxyl radical. The findings suggest that care should be exercised when simultaneous administration of either methyldopa or levodopa with ferrous sulphate is indicated.
Harkness JA, Blake DR. Penicillamine nephropathy and iron. Lancet 1982 Dec 18;2(8312):1368-1369.
Abstract: 7 of 16 patients with rheumatoid arthritis in whom penicillamine glomerulonephritis had developed had been taking oral iron, usually without the knowledge of their hospital clinician, while the dose of penicillamine was being gradually increased to an effective level. In 4 patients glomerulonephritis had appeared after the patients had stopped iron, with proteinuria developing with 2-5 months of discontinuation. Chelation of penicillamine by iron in the gut reduces its absorption, and in these 4 patients toxicity only became apparent after iron was stopped and there was a sudden increase in penicillamine absorption.
Hathcock JN. Metabolic mechanisms of drug-nutrient interactions. Fed Proc. 1985 Jan;44(1 Pt 1):124-129. (Review)
Hoffbrand AV, Wonke B. Iron chelation therapy. J Intern Med Suppl 1997;740:37-41.
Hoffbrand AV. Prospects for oral iron chelation therapy. J Lab Clin Med 1994 Apr;123(4):492-494. (Review)
Holt GA. Food and Drug Interactions. Chicago: Precept Press, 1998.
Hunt JR, Gallagher SK, Johnson LK. Effect of ascorbic acid on apparent iron absorption by women with low iron stores.
Am J Clin Nutr 1994;59:1381-1385.
Hussain MA, Green N, Flynn DM, Hoffbrand AV. Effect of dose, time, and ascorbate on iron excretion after subcutaneous desferrioxamine.
Lancet 1977 May 7;1(8019):977-979.
Abstract: The effect of 12 and 24 h continuous subcutaneous infusion of desferrioxamine (D.F.) on urinary iron excretion was compared in 13 patients with beta-thalassaemia major and 1 with congenital sideroblastic anaemia, all of whom were receiving regular blood-transfusions. 750 mg D.F. given over a 12 h period, gave a mean total (30 h) iron excretion of 17-5 mg, which was not statistically different from the mean iron excretion of 21-5 mg when the same dose was delivered over 24 h. 1500 mg D.F. gave a mean urinary iron excretion of 28-1 mg with a 12 h infusion, which was significantly less than the mean iron excretion of 39-6 mg with 24 h infusion. The 1500 mg dose gave a significant increase in iron excretion compared with the 750 mg dose when given by either 12 h or 24 h infusion. 7 of 8 patients, given D.F. over a 12 h period, had increased iron excretion when the dose was increased from 750 to 2000 mg. When the dose was increased to 4000 mg, however, the effect on iron excretion was variable. On the other hand, ascorbic-acid therapy was invariably associated with increased iron excretion after subcutaneous D.F. In twelve studies at different dose levels of D.F., ascorbate therapy was associated with increased iron excretion ranging from 24 to 245%. It is concluded that in most patients with transfusional iron overload subcutaneous D.F over a 12 h period, at a dose ranging from 2 to 4 g daily with ascorbic-acid saturation, is at present the most satisfactory method of removing excess iron.
Incalzi RA, Gemma A, Carbonin P. ACE inhibitors: a possible cause of unexplained anemia.
J Am Geriatr Soc. 1998 Jan;46(1):117-118. (Letter)
Kara M, Hasinoff BB, McKay DW, Campbell NR. Clinical and chemical interactions between iron preparations and ciprofloxacin.
Br J Clin Pharmacol 1991 Mar;31(3):257-261
Abstract: 1. The effect of ferrous sulphate (300 mg), ferrous gluconate (600 mg), and a combination tablet of iron (10 mg), magnesium (100 mg), zinc (15 mg), calcium (162 mg), copper (2 mg), and manganese (5 mg) (Centrum Forte) co-administration on ciprofloxacin bioavailability was tested in eight healthy subjects. 2. Peak serum ciprofloxacin concentrations and area under the curve (AUC) were significantly reduced when ciprofloxacin was administered with 300 mg ferrous sulphate (3.0 vs 2.0 mg l-1, P less than 0.05 and 12.3 vs 6.7 mg l-1 h, P less than 0.01, respectively). Reductions in peak ciprofloxacin concentrations and AUC also occurred when ciprofloxacin was ingested with 600 mg ferrous gluconate (1.3 mg l-1, P less than 0.01 and 4.1 mg l-1 h, P less than 0.01, respectively) and a Centrum Forte tablet (1.4 mg l-1, P less than 0.01 and 5.4 mg l-1 h, P less than 0.01, respectively). 3. When ferrous ion was mixed with ciprofloxacin, rapid spectral changes occurred (t1/2 = 1.9 min). Additional studies were consistent with oxidation of the ferrous form of iron to its ferric form, which is followed by rapid formation of a Fe(3+)-ciprofloxacin complex. Ciprofloxacin seems to bind to ferric ion in a ratio of 3:1 by interacting with the 4-keto and 3-carboxyl groups on ciprofloxacin. 4. The formation of a ferric ion-ciprofloxacin complex is probably the cause of the reduction in ciprofloxacin bioavailability in the presence of iron.
Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study.
Circulation. 1997 Nov 18;96(10):3300-3307.
Knodel LC, Talbert RL. Adverse effects of hypolipidaemic drugs. Med Toxicol 1987 Jan;2(1):10-32.
Abstract: Cholestyramine, colestipol, clofibrate, gemfibrozil, nicotinic acid (niacin), probucol, neomycin, and dextrothyroxine are the most commonly used drugs in the treatment of hyperlipoproteinaemic disorders. While adverse reaction data are available for all of them, definitive data regarding the frequency and severity of potential adverse effects from well-controlled trials using large numbers of patients (greater than 1000) are available only for cholestyramine, clofibrate, nicotinic acid and dextrothyroxine. In adult patients treated with cholestyramine, gastrointestinal complaints, especially constipation, abdominal pain and unpalatability are most frequently observed. Continued administration along with dietary manipulation (e.g. addition of dietary fibre) and/or stool softeners results in diminished complaints during long term therapy. Large doses of cholestyramine (greater than 32 g/day) may be associated with malabsorption of fat-soluble vitamins. Most significantly, osteomalacia and, on rare occasions, haemorrhagic diathesis are reported with cholestyramine impairment of vitamin D and vitamin K absorption, respectively. Paediatric patients have been reported to experience hyperchloraemic metabolic acidosis or gastrointestinal obstruction. Concurrent administration of acidic drugs may result in their reduced bioavailability. Serious adverse reactions to clofibrate will probably limit its role in the future. Of particular concern are ventricular arrhythmias, induction of cholelithiasis and cholecystitis, and the potential for promoting gastrointestinal malignancy which far outweigh the reported benefits in preventing new or recurrent myocardial infarction, cardiovascular death and overall death. Patients with renal disease are particularly prone to myositis, secondary to alterations in protein binding and impaired renal excretion of clofibrate. Drug interactions with coumarin anticoagulants and sulphonylurea compounds may produce bleeding episodes and hypoglycaemia, respectively. Nicotinic acid produces frequent adverse effects, but they are usually not serious, tend to decrease with time, and can be managed easily. Dermal and gastrointestinal reactions are most common. Truncal and facial flushing are reported in 90 to 100% of treated patients in large clinical trials. Significant elevations of liver enzymes, serum glucose, and serum uric acid are occasionally seen with nicotinic acid therapy. Liver enzyme elevations are more common in patients given large dosage increases over short periods of time, and in patients treated with sustained release formulations.
Koop H. Review article: metabolic consequences of long-term inhibition of acid secretion by omeprazole.
Aliment Pharmacol Ther 1992 Aug;6(4):399-406.
Abstract: Metabolic sequelae of profound and long-lasting inhibition of gastric acid secretion by omeprazole have largely been neglected. Data from long-term studies suggest that vitamin B12 stores decrease slightly over several years, although this was not clinically relevant within the first 4 years of therapy. Additionally, it cannot be completely ruled out that patients with an increased iron demand may develop iron deficiency, but data available at present do not provide any evidence that iron malabsorption is to be expected under normal conditions. Protein homeostasis and calcium metabolism seem to be unaffected by long-term omeprazole therapy. Based upon present experience, serum cobalamin concentration should be monitored in patients undergoing omeprazole therapy for several years.
Koop H, Bachem MG. Serum iron, ferritin, and vitamin B12 during prolonged omeprazole therapy.
J Clin Gastroenterol 1992 Jun;14(4):288-292.
Abstract: Since gastric acid plays an important role in the absorption process of iron and vitamin B12, we determined levels of iron, ferritin, vitamin B12, and folic acid in 75 serum samples obtained during continuous omeprazole therapy (6-48 months after start of therapy) from 34 patients with peptic diseases (primarily reflux esophagitis). Serum iron and ferritin levels were decreased in two and three patients, respectively, but there is little evidence that omeprazole administration was causally related to these findings. Serum vitamin B12 and folic acid levels were normal in all cases. We conclude that iron, vitamin B12, and folic acid malabsorption is unlikely to occur, at least within the initial 3-4 years of continuous omeprazole therapy.
Lehto P, Kivisto KT, Neuvonen PJ. The effect of ferrous sulphate on the absorption of norfloxacin, ciprofloxacin and ofloxacin.
Br J Clin Pharmacol 1994 Jan;37(1):82-85.
Abstract: The effect of ferrous sulphate on the absorption of norfloxacin, ciprofloxacin and ofloxacin was studied in three separate, two-period crossover trials, each involving eight healthy volunteers. After an overnight fast, a single dose of norfloxacin (400 mg), ciprofloxacin (500 mg) or ofloxacin (400 mg) was administered with and without ferrous sulphate (corresponding to 100 mg elemental iron). The absorption of all the fluoroquinolones studied was significantly reduced when they were co-administered with ferrous sulphate. The reduction in the area under the plasma drug concentration-time curve from 0 to 24 h was most marked in the case of norfloxacin, while ofloxacin was least affected by ferrous sulphate. The AUC of norfloxacin was reduced by 73% (P < 0.001) and its peak plasma concentration by 75% (P < 0.01) by concomitant ingestion of ferrous sulphate. The AUC and peak plasma concentration of ciprofloxacin were reduced by 57% (P < 0.001) and 54% (P < 0.01), respectively, by ferrous sulphate. Concomitant ingestion of ferrous sulphate reduced the AUC and peak plasma concentration of ofloxacin by 25% (P < 0.01) and 36% (P < 0.01), respectively. Similar results were obtained with respect to the urinary recoveries of each fluoroquinolone. We recommend that norfloxacin and ciprofloxacin should not be taken together with ferrous sulphate. It would also be advisable not to take ofloxacin with ferrous sulphate, especially if the organism causing infection is only moderately susceptible.
Leonards JR, Levy G, Niemczura R. Gastrointestinal blood loss during prolonged aspirin administration.
N Engl J Med. 1973 Nov 8;289(19):1020-1022.
Leyden JJ. Absorption of minocycline hydrochloride and tetracycline hydrochloride. Effect of food, milk, and iron.
J Am Acad Dermatol 1985 Feb;12(2 Pt 1):308-312.
Abstract: Serum concentrations of tetracycline hydrochloride and minocycline hydrochloride were compared when administered with water, milk, a meal, and 300 mg ferrous sulfate in two groups of eight volunteers. Absorption of both antibiotics was significantly decreased by administration with iron (77% inhibition with minocycline and 81% with tetracycline), milk (27% inhibition with minocycline, 65% with tetracycline), and food (13% inhibition with minocycline and 46% with tetracycline). The inhibitory effect on absorption with food and milk was significantly greater for tetracycline than for minocycline.
Lim, D, McKay, M. Food-drug interactions. Drug Information Bull 1995;15(2). (Review)
Lyle WH. Penicillamine and iron. Lancet 1976 Aug 21;2(7982):420. (Letter)
Machado FC, Demicheli C, Garnier-Suillerot A, Beraldo H. Metal complexes of anhydrotetracycline. 2. Absorption and circular dichroism study of Mg(II), Al(III), and Fe(III) complexes. Possible influence of the Mg(II) complex on the toxic side effects of tetracycline.
J Inorg Biochem 1995 Nov 15;60(3):163-173.
Marz R. Medical Nutrition From Marz. Second Edition. Portland, OR. 1997.
Masse PG, Roberge AG. Long-term effect of low-dose combined steroid contraceptives on body iron status.
Contraception 1992 Sep;46(3):243-252.
Abstract: The present study was aimed to evaluate iron metabolism in active and healthy adult women having taken oral contraceptives (OC) long-term. Mean dietary iron intake in age-matched control and experimental groups was adequate. Serum ferritin used as a marker for body iron stores was marginal in both groups underlying a high prevalence of deficient-iron reserves among subjects. This parameter was not correlated to the iron content of the diet. The serum iron concentration was significantly higher in OC users than control subjects (p less than 0.001). Biochemical results commanded a discussion on the pertinence of evaluating the total dietary iron intake and on the sensitivity of biochemical methods used to assess the iron status.
Mitjavila MT, Carbonell MT, Saiz MP, Muntane J, Sanchez J, Puig-Parellada P. Role of iron in carrageenan-induced granuloma: action of desferrioxamine and indometacin.
Pharmacology 1990;40(4):236-240.
Mooij PN, Thomas CM, Doesburg WH, Eskes TK. The effects of oral contraceptives and multivitamin supplementation on serum ferritin and hematological parameters.
Int J Clin Pharmacol Ther Toxicol 1992 Feb;30(2):57-62.
Abstract: We have studied the effects of oral contraceptive (OC) use and of subsequent multivitamin supplementation on several hematological parameters. Hemoglobin (Hb), hematocrit (Ht), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), and serum iron status (serum iron, total iron binding capacity and ferritin) were tested in groups of women with and without OC in view of preconceptional status. The group taking sub-50 OC comprised 28 women while 31 women were included in the group of non-OC users. Blood samples were taken on days 3 and 23 of the first cycle to obtain baseline values of each analyte. Multivitamin supplementation started on day 1 of the second cycle and this was continued daily throughout three consecutive cycles until the end of the study. Comparison of the baseline values of each parameter as determined on days 3 and 23 of the first cycle of the two groups revealed no significant different hematological parameters due to OC-use unlike the parameters of serum iron status which were all significantly increased for the group of OC-users as compared to the group of non-OC users. The effects of multivitamin supplementation on the hematological and iron status parameters were studied within each group. The consistency of each effect of multivitamin supplementation between the two groups was also tested. A multivitamin supplementation significantly decreased MCHC within either group, and caused increases of MCV, whereas Ht and MCH remained unaltered. With respect to the iron balance, serum iron and total iron binding capacity significantly increased, whereas serum ferritin decreased during multivitamin supplementation.
Oh VMS. Iron dextran and systemic lupus erythematosus. Br Med J 1992;305:1000. (Letter)
Osman MA, Patel RB, Schuna A, Sundstrom WR, Welling PG. Reduction in oral penicillamine absorption by food, antacid, and ferrous sulfate.
Clin Pharmacol Ther 1983 Apr;33(4):465-470.
Abstract: Plasma levels of penicillamine, urinary recovery of penicillamine and its oxidized metabolites, and urinary excretion of copper were examined after single 500-mg oral doses of penicillamine to six healthy men. Penicillamine was given after an overnight fast, a standard breakfast, and after antacid and ferrous sulfate. Following the fasting dose, the mean peak plasma level of 3.05 micrograms/ml developed at 3.8 hr and the drug was cleared from plasma with a t1/2 of 2.1 hr. Penicillamine levels were reduced to 52%, 35%, and 66% of those from the fasting dose after food, ferrous sulfate, and antacid. The rates of penicillamine appearance and disappearance from plasma were essentially treatment independent. There were good correlations between urinary recovery of total penicillamine (r = 0.875), between urinary copper excretion (r = 0.758) and the penicillamine plasma concentration AUCs. The availability of oral penicillamine is very susceptible to interactions with other substances. Further studies may be necessary to assess the full clinical significance of these interactions.
Ozbek N, Ozen S, Saatci U. Enalapril-induced anemia in a renal transplant patient.
Acta Paediatr Jpn. 1997 Oct;39(5):626-627.
Palomo I, Grebe G, Valladares G, Bustos P, Ferrada M. [Hemoglobin, serum iron and transferrin saturation among users of intrauterine devices and oral contraceptive agents].
Rev Med Chil 1990 May;118(5):506-511. [Article in Spanish]
Abstract: We studied 60 females using either intrauterine device or taking oral contraceptive pills. Hemoglobin, serum iron, total iron binding capacity and saturation of transferrin were determined before and 4 and 10 months after starting a responsible paternity program. Women with a basal hemoglobin level below 12 g/dl were excluded. Age, parity and hematologic parameters were similar for both groups. A significant decrease in hemoglobin level and saturation of transferrin was observed at 10 months in intrauterine device users (13.6 to 13.1 g/dl and 36.2 to 26.9%, respectively). Use of oral contraceptive pills was not associated to hemoglobin decrease but a significant rise in saturation of transferrin was observed (36.2 to 43.9%, p less than 0.05).
Pazzucconi F, Barbi S, Baldassarre D, Colombo N, Dorigotti F, Sirtori CR. Iron-ovotransferrin preparation does not interfere with ciprofloxacin absorption.
Clin Pharmacol Ther 1996 Apr;59(4):418-422.
Abstract: Iron supplements can interfere with the bioavailability of a number of drugs, including thyroxine, tetracycline derivatives, penicillamine, methyldopa, levodopa, carbidopa, ciprofloxacin, and the newer fluoroquinolones. A new iron formulation was tested in which iron ions are bound to ovotransferrin, a protein that shares more than an 80% similarity with the sequence of human transferrin and apparently is less likely than the commonly used iron salts to reduce drug absorption. Ciprofloxacin was taken as a model drug, of wide use and restricted range of therapeutic levels, and its absorption was evaluated after the administration of the iron-ovotransferrin complex versus an iron-gluconate formulation in healthy volunteers. At variance with the iron gluconate formulation, which led to a reduction of about 50% of peak serum ciprofloxacin levels (Cmax; 1.0 +/- 0.2 versus 2.4 +/- 0.3 micrograms/ml; p < 0.01) and of the area under the serum concentration-time curve from time 0 to infinity [AUC(0 - infinity); 10.1 +/- 1.1 versus 18.3 +/- 1.0 mg.L-1.hr; p < 0.01], the iron-ovotransferrin complex caused only modest, non significant changes in absorption with a minimal reduction of the AUC[0 - infinity) (17.3 +/- 1.0 versus 18.3 +/- 1.0 mg.L-1.hr; difference not significant) and a nonsignificant decrease in the Cmax (2.2 +/- 0.3 versus 2.4 +/- 0.3 microgram/ml; difference not significant). Iron was also well absorbed from the formulation in the presence of a fatty meal. The very common drug interactions with oral iron preparations can be effectively prevented by the use of the iron-ovotransferrin complex interacting to a minimal extent with a sensitive drug with a reduced margin of efficacy, such as ciprofloxacin.
Peterson DA, Gerrard JM, Rao GH, White JG. Inhibition of ferrous iron induced oxidation of arachidonic acid by indomethacin. Prostaglandins Med 1979 Feb;2(2):97-108.
Abstract: The molecular mechanism by which indomethacin exerts its inhibitory effects on the prostaglandin endoperoxide synthetase enzyme is unknown. In the present study we have explored the possibility that indomethacin might interact with Fe++ in the enzyme to produce its inhibitory effect. For this study we made use of the recent discovery that Fe++ alone can oxidize arachidonic acid, and the interaction of this fatty acid with the metal can be detected by following reduction of nitroblue tetrazolium (NBT) or by conversion of the Fe++ to Fe+++. Indomethacin markedly inhibited NBT reduction in the presence of arachidonic acid and Fe++ when the indomethacin had been preincubated with the Fe++. Indomethacin also inhibited the conversion of Fe++ to Fe+++ by arachidonic acid. Results obtained by varying the concentrations of indomethacin and arachidonic acid and measuring inhibition of the conversion of Fe++ to Fe+++ by the indomethacin are consistent with a one to one complex forming between indomethacin and Fe++. The complex between indomethacin and Fe++ separates on prolonged incubation of the complex with arachidonic acid. The nature of the binding is suggested by a molecular model. Our results suggest that indomethacin may act to inhibit the prostaglandin endoperoxide synthetase enzyme by complexing Fe++ in the enzyme. Ibuprofen and tolmetin, two other prostaglandin synthetase inhibitors, also inhibit the interaction of Fe++ with arachidonic acid suggesting this may be a general mechanism for this type of drug.
Polk RE. Drug-drug interactions with ciprofloxacin and other fluoroquinolones.
Am J Med 1989 Nov 30;87(5A):76S-81S.
Abstract: Early investigational trials with new quinolone antibiotics revealed two important drug-drug interactions: decreased fluoroquinolone absorption when co-administered with magnesium-aluminum antacids and inhibition of theophylline metabolism. Subsequent studies have investigated the mechanisms of these interactions. With respect to the effect of antacids, the absorption of all quinolones appears to be significantly reduced by antacids containing magnesium and/or aluminum, and concomitant administration must be avoided. Other cations, such as calcium, iron, and probably zinc, appear to interact in a similar manner. Chelation between the quinolone and cation is the most likely mechanism. With respect to the effect on theophylline metabolism, quinolones inhibit specific cytochrome P-450 isozymes responsible for metabolism of methylxanthines, although there are major differences between the quinolones. Enoxacin is the most potent inhibitor, followed by ciprofloxacin, pefloxacin, norfloxacin, and ofloxacin. Caffeine metabolism is also inhibited, although the clinical significance is uncertain. Case reports describe renal failure associated with concomitant administration of cyclosporine and ciprofloxacin, although controlled trials have not demonstrated an interaction. Enoxacin has little effect on warfarin metabolism, suggesting that other quinolones may not affect warfarin disposition. Case reports of central nervous system toxicity from administration of nonsteroidal anti-inflammatory agents and quinolones need confirmation. Patients should be monitored closely when potential interacting agents are used; it is probable that not all interactions have been identified.
Polk RE, Healy DP, Sahai J, Drwal L, Racht E. Effect of ferrous sulfate and multivitamins with zinc on absorption of ciprofloxacin in normal volunteers.
Antimicrob Agents Chemother 1989 Nov;33(11):1841-1844.
Abstract: Cations such as magnesium and aluminum significantly impair the absorption of ciprofloxacin. Twelve healthy adult male volunteers participated in this four-way crossover study to investigate the effects of ferrous sulfate and multivitamins with zinc on the absorption of ciprofloxacin. Doses of ciprofloxacin (500 mg) were given 7 days apart and after an overnight fast. Dose 1 was administered alone (regimen A). The subjects then received either a ferrous sulfate tablet (325 mg three times a day; regimen B) or a once-daily multivitamin with zinc (regimen C) for 7 days; dose 2 of ciprofloxacin was then given with the last dose of regimen B or C. Subjects were crossed over to the alternate regimen for 7 days, and dose 3 of ciprofloxacin was again administered with the last dose of regimen B or C. After a 7-day washout, dose 4 of ciprofloxacin was given (regimen D). Ciprofloxacin concentrations were determined by high-pressure liquid chromatography. The areas under the concentration-time curve (AUCs) of ciprofloxacin for regimens A and D were not significantly different (14.5 +/- 2.3 versus 15.7 +/- 2.8 micrograms.h/ml, mean +/- standard deviation). The AUCs for regimen B (5.4 +/- 1.7 micrograms.h/ml) and regimen C (11.3 +/- 2.4 micrograms.h/ml) were significantly different from the AUCs for regimens A and D. Peak concentrations of ciprofloxacin with regimen B were below the MIC for 90% of strains of many organisms normally considered susceptible. Ferrous sulfate and multivitamins with zinc significantly impaired the absorption of ciprofloxacin. The effect of ferrous sulfate is likely to be clinically significant; the responsible component of multivitamins with zinc requires additional study.
Powanda MC, Blackburn BS, Bostian KA, Fowler JP, Hauer EC, Pekarek RS. Clofibrate-induced alterations in zinc, iron and copper metabolism.
Biochem Pharmacol 1978 Jan 1;27(1):125-127.
Roe DA. Drug and nutrient interactions in the elderly diabetic. Drug Nutr Interact. 1988;5(4):195-203. (Review)
Roe DA. Drug-induced Nutritional Deficiencies. 2nd ed. Westport, CT: Avi Publishing, 1985.
Roe DA. Risk factors in drug-induced nutritional deficiencies. In: Roe DA, Campbell T, eds.
Drugs and Nutrients: The Interactive Effects. New York: Marcel Decker, 1984.
Sakagami H, Satoh K, Fukuchi K, Gomi K, Takeda M. Effect on an iron-chelator on ascorbate-induced cytotoxicity.
Free Radic Biol Med 1997;23(2):260-270.
Abstract: We investigated the effect of deferoxamine mesylate (DFO), an iron chelator, to test whether ascorbate-induced cytotoxicity is due to iron-catalyzed oxidation. Exposing human promyelocytic leukemic HL-60 cells to either sodium ascorbate or ascorbic acid for 1 h resulted in the progressive production of apoptotic cells characterized by cell shrinkage, as well as nuclear and internucleosomal DNA fragmentation. The addition of micromolar to millimolar concentrations of DFO during the 1-h exposure did not inhibit, but rather enhanced the ascorbate-induced apoptosis in both regular and serum-free RPMI1640 medium. However, a higher concentration of serum significantly inhibited the ascorbate-induced cytotoxicity. In contrast, the cytotoxic activity of ascorbate against T98G human glioblastoma cells was enhanced or reduced by micromolar and millimolar concentrations of DFO, respectively. Ascorbate significantly increased the oxidation potential in the culture medium, and the pro-oxidant action of ascorbate was further augmented by the presence of the cells. DFO did not significantly affect the ascorbyl radical intensity and only slightly reduced the ascorbate-elevated oxidation potential. These data demonstrated that ascorbate can induce cytotoxicity even in iron-deficient medium.
Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R. High stored iron levels associated with excess risk of myocardial infarction in western Finnish men.
Circulation 1992 Sep;86(3):803-811.
Abstract: BACKGROUND. Iron can induce lipid peroxidation in vitro and in vivo in humans and has promoted ischemic myocardial injury in experimental animals. We tested the hypothesis that high serum ferritin concentration and high dietary iron intake are associated with an excess risk of acute myocardial infarction. METHODS AND RESULTS. Randomly selected men (n = 1,931), aged 42, 48, 54, or 60 years, who had no symptomatic coronary heart disease at entry, were examined in the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) in Eastern Finland between 1984 and 1989. Fifty-one of these men experienced an acute myocardial infarction during an average follow-up of 3 years. On the basis of a Cox proportional hazards model adjusting for age, examination year, cigarette pack-years, ischemic ECG in exercise test, maximal oxygen uptake, systolic blood pressure, blood glucose, serum copper, blood leukocyte count, and serum high density lipoprotein cholesterol, apolipoprotein B, and triglyceride concentrations, men with serum ferritin greater than or equal to 200 micrograms/l had a 2.2-fold (95% CI, 1.2-4.0; p less than 0.01) risk factor-adjusted risk of acute myocardial infarction compared with men with a lower serum ferritin. An elevated serum ferritin was a strong risk factor for acute myocardial infarction in all multivariate models. This association was stronger in men with serum low density lipoprotein cholesterol concentration of 5.0 mmol/l (193 mg/dl) or more than in others. Also, dietary iron intake had a significant association with the disease risk in a Cox model with the same covariates. CONCLUSIONS. Our data suggest that a high stored iron level, as assessed by elevated serum ferritin concentration, is a risk factor for coronary heart disease.
Semba RD, Muhilal, West KP Jr, et al. Impact of vitamin A supplementation on hematological indicators of iron metabolism and protein status in children.
Nutr Res 1992;12:469-478.
Shakir KM, Chute JP, Aprill BS, Lazarus AA. Ferrous sulfate-induced increase in requirement for thyroxine in a patient with primary hypothyroidism.
South Med J 1997 Jun;90(6):637-639.
Abstract: Recent studies have shown that under experimental conditions ferrous sulfate may reduce the gastrointestinal absorption of orally administered levothyroxine sodium in patients with primary hypothyroidism. We describe a patient who became hypothyroid while taking ferrous sulfate. The hypothyroid status was corrected by increasing the dose of levothyroxine. Subsequently, when ferrous sulfate was discontinued, the patient became hyperthyroid while taking the higher dose of thyroid hormone preparation. Since both hypothyroidism and iron deficiency anemia may coexist, additional thyroid function testing is recommended in patients treated concurrently with ferrous sulfate and L-thyroxine.
Schlierf G, Vogel G, Kohlmeier M, Vuilleumier JP, Huppe R, Schmidt-Gayk H. [Long-term therapy of familial hypercholesterolemia in young patients with colestipol: availability of minerals and vitamins].
Klin Wochenschr. 1985 Sep 2;63(17):802-8026. [Article in German]
Abstract: Longterm treatment for 5 years of young patients with familial hypercholesterolemia was accompanied by monitoring of plasma levels of calcium, iron, sodium, parathyroid hormone and water- and fat soluble vitamins, since interference of the ion exchange resin with absorption of numerous substances as well as abnormal plasma levels of some of the above had been described in several studies. Treatment was effective in the group with good compliance (lowering of plasma cholesterol at the end of 5 years by 19% and, compared to the control group, by 23%). Adverse drug effects with respect to the above parameters were not found. Plasma levels of carotinoides and vitamin E were elevated in the patients according to elevated concentrations of lipoproteins which are carriers of these vitamins.
Steegers-Theunissen RP, Van Rossum JM, Steegers EA, Thomas CM, Eskes TK. Sub-50 oral contraceptives affect folate kinetics.
Gynecol Obstet Invest 1993;36(4):230-233.
Abstract: The effects of long-term use of oral contraceptives containing less than 50 micrograms of estrogen (sub-50 OCs) on the kinetics of folic acid monoglutamate, vitamin B12 levels, and iron status have been studied in 29 OC users (Marvelon) and in 13 women without OC use serving as controls. At 210 min after oral folate loading the median serum folate concentration was significantly lower in OC users when compared to the control group. OC users showed significantly higher total iron binding capacity and significantly lower serum vitamin B12 concentrations. This data demonstrates that sub-50 OCs significantly affect folate kinetics and vitamin B12 levels. However, the folate and vitamin B12 status does not seem to be at risk.
Stevens RG, Graubard BI, Micozzi MS, Neriishi K, Blumberg BS. Moderate elevation of body iron level and increased risk of cancer occurrence and death.
Int J Cancer 1994 Feb 1;56(3):364-369.
Stewart CA, Termanini B, Sutliff VE, Serrano J, Yu F, Gibril F, Jensen RT. Iron absorption in patients with Zollinger-Ellison syndrome treated with long-term gastric acid antisecretory therapy.
Aliment Pharmacol Ther 1998 Jan;12(1):83-98.
Abstract: BACKGROUND: Gastric acid secretion is important for absorption of dietary non-haem iron, and iron deficiency is common in gastric hyposecretory states such as after gastric resection. It is not known if prolonged, continuous treatment with potent acid suppressants such as omeprazole will lead to iron deficiency or lower body iron stores. AIM: To assess iron stores and the occurrence of iron deficiency anaemia in patients with Zollinger-Ellison syndrome (ZES) treated long-term with gastric antisecretory drugs. METHODS: One hundred and nine patients with ZES but without previous gastric resections were studied. All patients underwent assessment of acid control on antisecretory agents, determination of tumour extent, evaluation of haematological parameters (Hct, haemoglobin, WBC, MCV, MCHC), and determination of serum iron parameters (iron, ferritin, transferrin, iron/ transferrin ratio). Acid control values for the last 4 years were reviewed, the presence or absence of acid hyposecretion determined using three different criteria and this result correlated with haematological and iron parameters. RESULTS: Eighty-nine patients were taking omeprazole, nine patients were taking histamine H2-antagonists and 11 patients were taking no drugs following curative resection. The mean duration of omeprazole treatment was 5.7 years (range 0.7-12.5 years) and total duration of any treatment was 10.1 years (range 0.7-21 years). Acid hyposecretion was present by at least one criterion in 45% of patients. There were no significant differences between patients with or without acid hyposecretion, taking or not taking omeprazole, having different durations of omeprazole treatment or different durations of total time receiving any antisecretory treatment, for any serum iron parameter, haematological parameter, or for the frequency of iron deficiency. Males and females did not differ in percentage with low ferritin levels or percentage with iron deficiency. CONCLUSIONS: Continuous treatment with omeprazole for 6 years or continuous treatment with any gastric antisecretory drug for 10 years does not cause decreased body iron stores or iron deficiency. These results suggest that, in contrast to recent results which show yearly monitoring of vitamin B12 in such patients is needed, such monitoring for iron parameters is not necessary.
Suharno D, West CE, Muhilal, Karyadi D, Hautvast JG. Supplementation with vitamin A and iron for nutritional anemia in pregnant women in West Java, Indonesia.
Lancet 1993;342:1325-1328.
Sullivan JL. Stored iron and ischemic heart disease. Circulation 1992;86:1036. (Editorial)
Taberner DA. Iron deficiency and stanozolol therapy. Lancet 1983 Mar 19;1(8325):648. (Letter)
Taylor RT, Huskisson EC, Whitehouse GH, Hart FD, Trapnell DH. Gastric ulceration occurring during indomethacin therapy.
Br Med J 1968 Dec 21;4(633):734-737.
Threlkeld DS, ed. Hormones, Thyroid Hormones. In: Facts and Comparisons Drug Information. St. Louis, MO: Facts and Comparisons, Jun 1991.
Threlkeld DS, ed. Miscellaneous Products, Penicillamine. In: Facts and Comparisons Drug Information. St. Louis, MO: Facts and Comparisons, Aug 1996.
Trovato A, Nuhlicek DN, Midtling JE. Drug-nutrient interactions. Am Fam Physician 1991 Nov;44(5):1651-1658.(Review)
Tzonou A, Lagiou P, Trichopoulou A, Tsoutsos V, Trichopoulos D. Dietary iron and coronary heart disease risk: a study from Greece.
Am J Epidemiol 1998;147:161-166.
USDA: Composition of Foods. USDA Handbook #8. Washington DC, ARS, USDA, 1976-1986.
Watkins DW, Khalafi R, Cassidy MM, Vahouny GV. Alterations in calcium, magnesium, iron, and zinc metabolism by dietary cholestyramine.
Dig Dis Sci 1985 May;30(5):477-482.
Abstract: Cholestyramine is an effective drug for the reduction of plasma cholesterol because of its ability to sequester intestinal bile acids. Since metabolic alterations, including diminished intestinal absorption of vitamin D and osteomalacia have been reported with long-term use of this resin, the influence of cholestyramine on dietary balance of four mineral elements has been investigated. Wistar-strain rats were fed either a 2% cholestyramine or control diet for one month. Dietary intakes and fecal and urinary excretions of calcium, magnesium, iron, and zinc were determined using atomic absorption spectrophotometry during three, 3-day balance periods. Cholestyramine-fed rats had a net negative balance for calcium and a lower net positive balance for magnesium, iron, and zinc than the controls. Other effects of cholestyramine were an increased urinary excretion of calcium and magnesium, a decreased urinary zinc, and an alkalinization of urine. Blood and tissue cation content was unchanged except for a reduction in serum magnesium with resin feeding. Alterations in calcium, magnesium, and zinc metabolism might be explained by inadequate vitamin D absorption from the intestine followed by an increased secretion of parathyroid hormone. A diminished iron absorption due to resin binding could account for the reported disturbance in iron balance.
Werbach MR. Foundations of Nutritional Medicine. Tarzana, CA: Third Line Press, 1997, 221-222. (Review).
Wong KC, Woo KS, Lam WK, Li KT, Lai KN, Nicholls MG, Lui SF. A comparison of the effect of enalapril and metoprolol on renal function, potassium balance, lipid profile, cardiac function, exercise tolerance and quality of life in hypertensive dialysis patients.
Int J Artif Organs 1995 Dec;18(12):757-762.
Abstract: 26 patients with hypertension while on hemodialysis or continuous ambulatory peritoneal dialysis for end-stage renal diseases were treated first with enalapril and then changed to metoprolol. Both drugs were shown to be similarly effective in controlling blood pressure. There was no difference between the two drugs in their effects on renal function, potassium balance, lipid profile, cardiac function, exercise tolerance, and quality of life. Mild worsening of anemia was observed during treatment with enalapril. No serious side effects were reported. Use of enalapril is safe in the treatment of hypertension in dialysis patients.