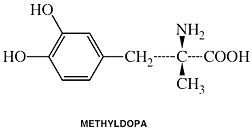
Methyldopa
Brand Names: Aldomet
Clinical Names: Methyldopa
Summary
generic name: Methyldopa
trade name: Aldomet®
type of drug: Antihypertensive
used to treat: Arterial hypertension.
overview of interactions:
• nutrient affecting drug performance: Iron
• adverse interaction: Sodium
• interference or depletion due to drug: Vitamin B12 (Cobalamin)
• dietary factor affecting drug performance: Food, especially Dietary Protein
Interactions
nutrient affecting drug performance: Iron
• mechanism: Research has consistently found that iron, in several forms, binds strongly to methyldopa, producing iron complexes, and thereby reduces the availability of methyldopa and hence its absorption.
• research: In one small study, four of five patients using methyldopa who were put on ferrous sulfate experienced an increase in blood pressure within two weeks.
(Campbell N, et al. Clin Pharmacol Ther 1988 Apr;43(4):381-386)
• nutritional concerns: Individuals who have been prescribed methyldopa should avoid supplementing with iron without first consulting with their prescribing physician and/or a nutritionally-oriented healthcare professional. In general, this interactions can be avoided, or at least minimized, by taking melthyldopa two hours before or after iron-containing products.
adverse interaction: Sodium
• mechanism: Methyldopa is intended to reduce blood pressure. Consequently, limiting dietary sodium (salt) intake is advisable since high levels can contribute to fluid retention and thereby interfere with the intended action of methyldopa in lowering blood pressure.
• nutritional support: The simplest way to avoid this counterproductive effect is to reduce the consumption of table salt and heavily salted foods while using methyldopa for the treatment of hypertension.
interference or depletion due to drug: Vitamin B12
(Cobalamin)
• mechanism: Methyldopa use can reduce vitamin B12 levels, potentially leading to a vitamin B12 deficiency.
• nutritional support: Supplementation of vitamin B12 at moderate levels of 10-25 mcg per day can counteract this tendency to depletion with no significant risk of adverse effects.
dietary factor affecting drug performance: Food, especially Dietary Protein
• mechanism: Eating meals, especially those high in protein, too close to taking methyldopa can interfere with its absorption and action.
(Stenbaek O, et al. Acta Pharmacol Toxicol (Copenh). 1982 Mar;50(3):225-229; Sved AF, et al.
J Pharmacol Exp Ther. 1980 Jul;214(1):147-151.)
• nutritional support: As a precaution individuals on methyldopa therapy should avoid taking the drug less than one hour before or two hours after eating to reduce the risk of this interference.


Please read the disclaimer concerning the intent
and limitations of the information provided here.
Do not rely solely on the information in this article.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
References
Campbell N, Paddock V, Sundaram R. Alteration of methyldopa absorption, metabolism, and blood pressure control caused by ferrous sulfate and ferrous gluconate.
Clin Pharmacol Ther 1988 Apr;43(4):381-386.
Abstract: This study examined the effect of two widely used iron treatments on methyldopa absorption, metabolism, and blood pressure control. A 500 mg tablet of methyldopa (2.37 mmol) was taken with and without ferrous sulfate (325 mg) by 12 normal subjects in a randomized crossover trial. When ferrous sulfate was taken with methyldopa there was a decrease in the proportion of methyldopa excreted as "free" methyldopa (49.5% +/- 12.4% vs 21.1% +/- 4.77%; p less than 0.01), a significant increase in the proportion excreted as methyldopa sulfate (37.8% +/- 12.3% vs 65.8% +/- 10.5%; p less than 0.01), and a decrease in the percentage of methyldopa absorbed (29.1% +/- 12.5% vs 7.88% +/- 4.14%; p less than 0.01). These factors resulted in an 88% reduction in the quantity of "free" methyldopa excreted. To determine if an iron preparation without sulfate produced the same effect, the study was repeated with ferrous gluconate (600 mg) with similar results. The clinical consequences of the methyldopa-ferrous sulfate interaction was determined in five hypertensive subjects receiving chronic methyldopa therapy. The subjects took ferrous sulfate for 2 weeks. There was an increase in both systolic and diastolic blood pressure in four patients and a decrease in blood pressure in all patients after ferrous sulfate was discontinued. The increases in blood pressure were substantial in three of the patients.
Campbell, NR, Hasinoff, BB. Iron supplements: A common cause of drug interactions.
Br J Clin Pharmacol 1991 Mar;31(3):251-255. (Review)
Abstract: Iron-drug interactions of clinical significance may occur in many patients and involve a large number of therapies. Concurrent ingestion of iron causes marked decreases in the bioavailability of a number of drugs. The affected drugs, tetracycline, tetracycline derivatives (doxycycline, methacycline and oxytetracycline), penicillamine, methyldopa, levodopa, carbidopa and ciprofloxacin have diverse chemical structures and clinical effects. The major mechanism of these drug interactions is the formation of iron-drug complexes (chelation or binding of iron by the involved drug). A large number of other important and commonly used drugs such as thyroxine, captopril and folic acid have been demonstrated to form stable complexes with iron. There is little known about the effects of concurrent therapy with iron supplements for most of the drugs.
Campbell NR, Campbell RR, Hasinoff BB. Ferrous sulfate reduces methyldopa absorption: methyldopa: iron complex formation as a likely mechanism.
Clin Invest Med 1990 Dec;13(6):329-332.
Abstract: Ferrous sulfate and sodium sulfate reduce methyldopa absorption in humans. This current study was conducted to investigate some of the potential factors by which these compounds could reduce methyldopa absorption. A rat model developed to examine drug absorption was used. Solutions of 14C methyldopa alone and with ferrous sulfate or sodium sulfate were injected in vivo into closed duodenal segments. Ferrous sulfate reduced methyldopa absorption 52.9% (p less than 0.01), while sodium sulfate had no significant effect on methyldopa absorption. In vitro iron in its ferrous form rapidly oxidizes to the ferric form in the presence of methyldopa. The ferric form of iron binds strongly to methyldopa, presumably resulting in the decreased methyldopa absorption. Methyldopa was stable in vivo and in vitro in the presence of ferrous sulfate and sodium sulfate. These studies are consistent with ferrous sulfate reducing methyldopa absorption by the formation of ferric iron: methyldopa complexes.
Greene RJ, Hall AD, Hider RC. The interaction of orally administered iron with levodopa and methyldopa therapy.
J Pharm Pharmacol 1990 Jul;42(7):502-504.
Abstract: The ability of methyldopa and levodopa to interact with both ferrous and ferric iron under a variety of conditions likely to be encountered physiologically has been examined. Spectrophotometric studies of ferrous sulphate in the presence of methyldopa indicate that no complexation occurs below pH2, whilst between pH 4-9, a variety of iron-methyldopa complexes is formed. The formation of these complexes is fast at high pH (pH 9: t1/2 less than 5 s), whilst the rate slows as the pH is lowered (pH 4: t1/2 greater than 30 min). These complexes are characteristic of iron-catecholate species, indicating that in the presence of methyldopa (and levodopa) ferrous iron undergoes autoxidation to the ferric form. The tight binding of ferric iron to methyldopa is predicted to alter the biodistribution characteristics of the complex with respect to the unchelated components. Furthermore, under the acid conditions of the stomach, redox cycling can occur. This will result in both catechol oxidation and production of the toxic hydroxyl radical. The findings suggest that care should be exercised when simultaneous administration of either methyldopa or levodopa with ferrous sulphate is indicated.
Holt GA. Food and Drug Interactions. Chicago: Precept Press, 1998, 74, 170-172.
Stenbaek O, Myhre E, Rugstad HE, Arnold E, Hansen T. The absorption and excretion of methyldopa ingested concomitantly with amino acids or food rich in protein.
Acta Pharmacol Toxicol (Copenh). 1982 Mar;50(3):225-229.
Abstract: Absorption and urinary excretion of 2-14C-L-alpha-methyldopa (alpha MD) were investigated in 5 healthy human volunteers. The drug was administered alone, with a mixture of amino acids and with a meal consisting of roast beef. The radioactivity in plasma and urine was not decreased significantly by either treatment, and it was concluded that the overall absorption of the drug was virtually unchanged. The amount of unconjugated alpha MD in plasma and urine, however, was significantly decreased following administration of the drug with roast beef. As the acid labile conjugates of alpha MD were practically unchanged an increased biotransformation to other metabolites owing to the protein rich meal was suggested more probable than competition between alpha MD and amino acids in the specific transport systems.
Sved AF, Goldberg IM, Fernstrom JD. Dietary protein intake influences the antihypertensive potency of methyldopa in spontaneously hypertensive rats.
J Pharmacol Exp Ther. 1980 Jul;214(1):147-151.
Abstract: When spontaneously hypertensive rats consumed a single meal before receiving methyldopa (MD), the increase in brain MD levels and the blood pressure reduction varied inversely with the meal's protein content. MD injection elicited a greater reduction in blood pressure (and a larger rise in brain MD) in spontaneously hypertensive rats consuming a protein-free meal than in fasting animals; it induced a smaller fall in blood pressure (and a smaller increase in brain MD) in spontaneously hypertensive rats ingesting a protein-containing meal than in fasting animals. This effect may depend on the large neutral amino acids contained in protein. Ingestion of a food identical in amino acid content to 18% casein, but containing an amino acid mixture instead of protein, also blunted the MD-induced fall in blood pressure. However, consumption of a similar diet, lacking the large neutral amino acids (but not other amino acids), elicited an MD-induced blood pressure reduction similar to that caused by ingesting a protein-free diet. The combined effects of meal consumption and MD injection on brain MD and blood pressure correlated significantly with the serum ration of MD to the sum of the natural large neutral amino acids and not with serum MD alone. Similar effects were also noted with chronic dietary and MD treatments. Thus, dietary protein content can influence significantly the potency of a clinically important amino acid drug that acts within the brain.

