
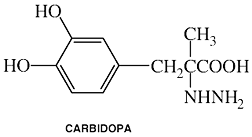
Brand Names: Atamet; Sinemet
Clinical Names: Carbidopa
Summary
generic name: Carbidopa
trade name: Atamet®
related drug: Levodopa-carbidopa (Sinemet®) is a combination of two drugs, levodopa and carbidopa.
used to treat: Management of symptoms of Parkinson's disease.
mechanism: Parkinson's disease is believed to be related to low levels of dopamine in certain parts of the brain. When levodopa is taken orally, it crosses through the "blood-brain barrier." Once it crosses the BBB, it is converted to dopamine. The resulting increase in brain dopamine concentrations is believed to improve nerve conduction and assist the movement disorders in Parkinson's disease. Carbidopa does not cross the blood-brain barrier. Carbidopa is added to the levodopa to prevent the breakdown of levodopa before it crosses into the brain. The addition of carbidopa allows lower doses of levodopa to be used. This reduces the risk of side effects from levodopa such as nausea and vomiting.
overview of interactions:
• nutrient affecting drug performance: Iron
• drug-nutrient interaction: L-5 Hydroxytryptophan (5-HTP)
• possible herb affecting drug toxicity: Hypericum perforatum
(St. John's Wort)
Interactions
nutrient affecting drug performance: Iron
• mechanism: Iron supplements taken with carbidopa interfere with the action of the drug.
(Campbell NR, Hasinoff BB. Brit J Clin Pharmacol 1991;31:251-255; Campbell NR, et al.
Br J Clin Pharmacol 1990 Oct;30(4):599-605.)
• nutritional concerns: Individuals using carbidopa should not take iron supplements without consulting with the prescribing physician and/or their pharmacist.
drug-nutrient interaction: L-5 Hydroxytryptophan(5-HTP)
• research: Some studies have found that the combination of 5-HTP and carbidopa 5-HTP and carbidopa may have a beneficial effect in the treatment of intention myoclonus, a neuromuscular disorder. Other research has not been able to confirm these outcomes.
(Growdon JH, et al. Neurology 1976;26:1135-1140; Van Woert MH, et al. N Engl J Med 1977;296:70-75; Magnussen I, et al.
Acta Neurol Scand 1978;57:289-294; Magnussen I, et al. Acta Pharmacol Toxicol (Copenh). 1981 Sep;49(3):184-189; Magnussen I, Van Woert MH.
Eur J Clin Pharmacol. 1982;23(1):81-86; Van Woert MH. Prog Clin Biol Res. 1983;127:43-52.)
• reports: Sternberg et al reported a scleroderma-like illness which developed in a patient treated with L-5 hydroxytryptophan (L-5HTP) and carbidopa for intention myoclonus. The patient had high plasma kynurenine levels that remained high when the L-5HTP-carbidopa combination was discontinued and rose again when the drug was reintroduced, suggesting that the drug unmasked an abnormality in one of the enzymes that catabolize kynurenine. They concluded that high plasma serotonin and the abnormality associated with elevated kynurenine were important in the pathogenesis of some scleroderma-like illnesses. Other researchers have published similar reports.
(Sternberg EM, et al. N Engl J Med 1980 Oct 2;303(14):782-787; Auffranc JC, et al.
Ann Dermatol Verereol 1985;112:691-692; Joly P, et al. J Am Acad Dermatol 1991;25:332-333.)
• nutritional concerns: Until further research is conducted into the interactions of carbidopa and 5-HTP individuals taking carbidopa should refrain from taking 5-HTP as a supplement unless they have consulted with and are under the supervision of a physician trained in nutritional therapies.
possible herb affecting drug toxicity: Hypericum perforatum (St. John's Wort)
• mechanism: Use of Sinemet® (levodopa-carbidopa) with monoamine oxidase inhibitors (MAOI's) antidepressants, such as isocarboxazid, phenelzine (Nardil®), tranylcypromine (Parnate®), and procarbazine (Matulane®), can result in severe and dangerous elevations in blood pressure.
• herbal concern: Pharmaceutical MAOI's are usually discontinued 2-4 weeks before starting Sinemet® (levodopa-carbidopa) usage. Since it is has been proposed that Hypericum acts in a manner similar to MAO-inhibitors, similar caution would be advised with regard to a potential interaction pending more conclusive research findings.

![]()
Please read the disclaimer concerning the intent and limitations of the information provided here.
Do not rely solely on the information in this article.
The information presented in Interactions is for informational and educational purposes only. It is based on scientific studies (human, animal, or in vitro), clinical experience, case reports, and/or traditional usage with sources as cited in each topic. The results reported may not necessarily occur in all individuals and different individuals with the same medical conditions with the same symptoms will often require differing treatments. For many of the conditions discussed, treatment with conventional medical therapies, including prescription drugs or over-the-counter medications, is also available. Consult your physician, an appropriately trained healthcare practitioner, and/or pharmacist for any health concern or medical problem before using any herbal products or nutritional supplements or before making any changes in prescribed medications and/or before attempting to independently treat a medical condition using supplements, herbs, remedies, or other forms of self-care.
References
Auffranc JC, Berbis P, Fabre JF, Garnier JP, Privat Y. Sclerodermiform and poikilodermal syndrome observed during treatment with carbidopa and 5-hydroxytryptophan. Ann Dermatol Venereol. 1985;112(9):691-692. [Article in French]
Campbell NR, Hasinoff BB. Iron supplements: A common cause of drug interactions.
Brit J Clin Pharmacol 1991 Mar;31(3):251-255. (Review)
Abstract: Iron-drug interactions of clinical significance may occur in many patients and involve a large number of therapies. Concurrent ingestion of iron causes marked decreases in the bioavailability of a number of drugs. The affected drugs, tetracycline, tetracycline derivatives (doxycycline, methacycline and oxytetracycline), penicillamine, methyldopa, levodopa, carbidopa and ciprofloxacin have diverse chemical structures and clinical effects. The major mechanism of these drug interactions is the formation of iron-drug complexes (chelation or binding of iron by the involved drug). A large number of other important and commonly used drugs such as thyroxine, captopril and folic acid have been demonstrated to form stable complexes with iron. There is little known about the effects of concurrent therapy with iron supplements for most of the drugs.
Campbell NR, Rankine D, Goodridge AE, Hasinoff BB, Kara M. Sinemet-ferrous sulphate interaction in patients with Parkinson's disease.
Br J Clin Pharmacol 1990 Oct;30(4):599-605.
Abstract: 1. This study examined the effects of administering ferrous sulphate 325 mg with Sinemet (100/25 tablet) on levodopa and carbidopa bioavailability and on signs of Parkinson's disease in nine patients. 2. Ferrous sulphate ingestion with Sinemet resulted in a decrease in levodopa area under the curve (AUC) of 30% (P less than 0.01) and a greater than 75% decrease in carbidopa AUC. Despite a strong relationship between reductions in levodopa AUC and reductions in Sinemet efficacy (r = 0.83, P less than 0.01), the average reduction in Sinemet's efficacy associated with ferrous sulphate did not achieve statistical significance (P = 0.055). 3. Chemical studies indicate that iron forms chemical complexes with carbidopa in a similar manner to levodopa and is a likely mechanism for the drug interactions. 4. AUC when a Sinemet tablet is taken concurrently with a ferrous sulphate tablet appears to be clinically significant in some but not all patients. The clinical significance of repeated ingestion of ferrous sulphate with Sinemet requires further studies.
Growdon JH, Young RR, Shahani BT. L-5-hydroxytryptophan in treatment of several different syndromes in which myoclonus is prominent.
Neurology 11976 Dec;26(12):1135-1140.
Abstract: The serotonin precursor L-5-hydroxytryptophan is useful therapy for patients with posthypoxic intention myoclonus. L-5-hydroxytryptophan plus carbidopa was administered to eight patients with this disorder or other syndromes in which myoclonus is prominent. This treatment (1) decreased the frequency of occurrence and amplitude of intention myoclonus in two patients with posthypoxic intention myoclonus and in one with idiopathic myoclonus, (2) had no effect in one patient with congenital encephalopathy and myoclonus, and (3) increased the frequency of occurrence and amplitude of myoclonus in two patients with lipid storage disease, one with myoclonic epilepsy, and in an additional patient with idiopathic myoclonus. Therefore, L-5-hydroxytryptophan does not effect improvement in all forms of myoclonus; it should be given with caution because it produces a high incidence of side effects. A patient's response to L-5-hydroxytryptophan therapy may be important in a diagnostic classification of myoclonic syndromes based on differences in indoleamine neurotransmitter function.
Joly P, Lampert A, Thromine E, Lauret P. Development of pseudobullous morphea and scleroderma-like illness during therapy with L-5-hydroxytryptophan and carbidopa. J Am Acad Dermatol. 1991 Aug;25(2 Pt 1):332-333.
Magnussen I, Nielsen-Kudsk F. Bioavailability and related pharmacokinetics in man of orally administered L-5-hydroxytryptophan in steady state.
Acta Pharmacol Toxicol (Copenh). 1980 Apr;46(4):257-262.
Abstract: The bioavailability of orally administered L-5-hydroxytryptophan in steady state was investigated at four increasing multiple dose levels in five patients suffering from various myoclonic disorders. An L-aromatic amino acid decarboxylase inhibitor was co-administered in all the experiments. The disposition pharmacokinetics of the amino acid had been established in the same patients in preceding intravenous single dose experiments. The finding of a direct proportionality between the size of the oral dose level of L-5-hydroxytryptophan and the corresponding areas under the plasma concentration curves within a dosage interval at steady state strongly indicates dose independent, linear pharmacokinetics of the compound. The systemic availability of L-5-hydroxytryptophan exhibited an interindividual range of 47-84%, with a mean value of 69.2% +/- 4.7 S.E.M. The absorption took place at a rather slow rate as judged from times of 1.8 to 3.3 hours elapsing from administration of the compound until occurance of the maximum measured plasma concentrations. Transitory nausea and vomiting were only recognized in few instances during the gradual building up of increasing steady state levels of L-5-hydroxytryptophan in the patients, and the importance of a slow initiation of therapeutical treatment with the amino acid is emphasized.
Magnussen I, Van Woert MH. Human pharmacokinetics of long term 5-hydroxytryptophan combined with decarboxylase inhibitors.
Eur J Clin Pharmacol. 1982;23(1):81-86.
Abstract: L-5-Hydroxytryptophan (5HTP) and its major metabolites 5-hydroxytryptamine (5HT) and 5-hydroxyindoleaceticacid (5HIAA) were measured in blood and cerebrospinal fluid from neurological patients receiving steady state treatment with 5HTP. There was accumulation of 5HT in blood platelets and 5HIAA in plasma in all patients, despite concomitant administration of the L-aromatic amino acid decarboxylase inhibitors, carbidopa and benserazide. There was no correlation between the 5HTP dose and the circulating concentrations of the aminoacid or its metabolites. Preliminary comparison of the biochemical and therapeutic effects of carbidopa versus benserazide suggest that 5HTP: carbidopa is superior to 5HTP: benserazide. A direct proportionality between plasma 5HTP concentrations and the levels of 5HTP in the lumbar cerebrospinal fluid was found. The binding to serum proteins of 5HTP in the clinically relevant concentration range of 10 to 100 microM was investigated; 19% of circulating 5HTP was bound to serum proteins. 5HTP did not displace protein-bound tryptophan in serum.
Magnussen I, Jensen TS, Rand JH, Van Woert MH. Plasma accumulation of metabolism of orally administered single dose L-5-hydroxytryptophan in man.
Acta Pharmacol Toxicol (Copenh). 1981 Sep;49(3):184-189.
Abstract: Single oral doses of L-5-hydroxytryptophan (5-HTP) were administered in combination with L-aromatic amino acid decarboxylase inhibitors. The time courses of plasma concentrations of 5-HTP, 5-hydroxytryptamine (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) and the concentrations of 5-HT in blood platelets were measured. Carbidopa enhanced the rise in plasma concentrations of 5-HTP 5-15 fold and counteracted the increase in plasma 5-HIAA levels induced by 5-HTP alone. A single dose of the decarboxylase inhibitor was equipotent to 14 days' pretreatment. Plasma or platelet concentrations of 5-HT failed to reflect the metabolism of 5-HTP. The ratio of 5-HTP to carbidopa influenced the systemic bioavailability of single dose administered 5-HTP indicating dose dependent absorption kinetics. Co-administration of L-dopa with 5-HTP and decarboxylase inhibitors had no effect on gastrointestinal absorption of 5-HTP in six parkinsonian patients.
Magnussen I, Dupont E, Engbaek F, de Fine Olivarius B. Post-hypoxic intention myoclonus treated with 5-hydroxytryptophan and an extracerebral decarboxylase inhibitor.
Acta Neurol Scand 1978 Apr;57(4):289-294.
Abstract: Post-hypoxic intention myoclonus successfully treated by long-term administration of the combination of 5-hydroxytryptophan and carbidopa is described. Persistent euphoria and diarrhoea were essential side effects. Methysergide (12 mg/day) blocked the therapeutic effect, indicating a specific serotoninergic function of precursor loading with 5-hydroxytryptophan. Tryptophan (8 g/day) had no effect on the myoclonus suggesting a reduced tryptophan hydroxylase activity. Plasma concentrations of 5-hydroxytryptophan in the range of 10--30 micromoles per liter were obtained during maintenance therapy with 900 mg 5-hydroxytryptophan per day in combination with 150 mg carbidopa per day.
Sternberg EM, Van Woert MH, Young SN, Magnussen I, Baker H, Gauthier S, Osterland CK. Development of a scleroderma-like illness during therapy with L-5-hydroxytryptophan and carbidopa.
New Engl J Med 1980 Oct 2;303(14):782-787.
Abstract: A scleroderma-like illness developed in a patient treated with L-5 hydroxytryptophan (L-5HTP) and carbidopa for intention myoclonus. The patient had high plasma kynurenine levels that remained high when the L-5HTP-carbidopa combination was discontinued, However, levels rose futher on drug rechallenge, suggesting that the drug unmasked an abnormality in one of the enzymes that catabolize kynurenine. Plasma kynurenine was also determined to be high in seven of 15 patients wth idiopathic scleroderma, but not in eight patients with intention myoclonus treated with L-5HTP and a decarboxylase inhibitor and in whom scleroderma did not develop or in 10 patients with Parkinson's disease treated wth L-dopa and carbidopa. Our data and studies in the literature suggest that two factors may be important in the pathogenesis of some scleroderma-like illness: high plasma serotonin and the abnormality associated with elevated kynurenine.
Van Woert MH, Rosenbaum D, Howieson J, Bowers MB Jr. Long-term therapy of myoclonus and other neurologic disorders with L-5-hydroxytryptophan and carbidopa.
N Engl J Med. 1977 Jan 13;296(2):70-75.
Abstract: We evaluated the therapeutic effect of L-5-hydroxytryptophan (L-5HTP), the precursor of serotonin (5-hydroxytryptamine), combined with carbidopa, a peripheral decarboxylase inhibitor, in patients with intention myoclonus and examined the serotonin metabolites in spinal fluid, blood and urine before and during therapy. In 18 patients with intention myoclonus due to anoxia or other brain damage, 11 derived more than 50% overall improvement during treatment with L-5HTP and carbidopa. Spinal-fluid 5-hydroxyindoleacetic acid was 35% lower in patients with intention myoclonus than in controls (P less than 0.05). Therapy with L-5HTP and carbidopa increased the concentration of serotonin metabolites in urine and spinal fluid. We postulate that a deficiency of brain serotonin is causally related to intention myoclonus and that the therapeutic effect of L-5HTP and carbidopa may be due to the repletion of serotonin in regions of the brain where serotoninergic neurons have degenerated.
Van Woert MH. Myoclonus and L-5-hydroxytryptophan (L-5HTP). Prog Clin Biol Res. 1983;127:43-52.