
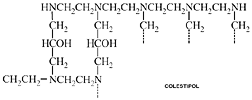
Colestipol
Brand Names: Colestid
Clinical Names: Colestipol
Summary
generic name: Colestipol
trade name: Colestid®
type of drug: Bile acid sequestrant.
used to treat: Reduction of elevated serum cholesterol in patients with primary
hypercholesterolemia (elevated low density lipoproteins); largely replaced by other drugs.
overview of interactions:
• nutrients affected by drug: Vitamin A, Vitamin D, Vitamin E, Vitamin K and Folate
• nutrient affected by drug: Calcium
• nutrient affected by drug: Iron
• nutritional concern: Constipation
Interactions
• nutrients affected by drug: Vitamin A, Vitamin D, Vitamin E, Vitamin K and Folate
• mechanism: Colestipol, by design, reduces fat absorption and hence interferes with absorption of fat-souble nutrients.
(Tonstad S, et al. Arch Dis Child 1996 Feb;74(2):157-160.)
• clinical concerns: This interaction raises serious questions given the accumulating experimental, epidemiological, and clinical evidence of an association between anti-oxidant vitamin intake (especially vitamin E) and reduced risk of coronary heart disease.
• research: Hodis et al have reported an association between supplementary vitamin E intake and angiographically demonstrated reduction in coronary artery lesion progression. Tonstad et al conducted a study of 37 boys and 29 girls aged 10-16 years with familial hypercholesterolemia first in an eight week double blind, placebo controlled protocol, then in open treatment for 44-52 weeks. They found that levels of serum folate, vitamin E, and carotenoids were reduced in the colestipol group. After one year of colestipol, those who took 80% or more of the prescribed dose had a greater decrease in serum 25-hydroxyvitamin D levels than those who took less than 80%.
(Hodis HN, et al. JAMA 1995 Jun 21;273(23):1849-1854; Tonstad S, et al. Arch Dis Child 1996 Feb;74(2):157-160.)
• related research: While supplementary Vitamin E has been found to be effective in treating high cholesterol by reducing progression of carotid arterial wall intima-media thickness, there has been no confirmation that the addition of moderate doses of Vitamin E along with colestipol will enhance the effectiveness of the therapy in reducing the progression of atherosclerosis.
(Azen SP, et al. Circulation 1996 Nov 15;94(10):2369-2372; Schlierf G, et al.
Klin Wochenschr. 1985 Sep 2;63(17):802-8026; Schwarz KB, et al. Pediatrics. 1980 Feb;65(2):243-250; Leonard JP, et al.
Arzneimittelforschung 1979;29(7):979-981; Tsang RC, et al. Pediatr Res. 1978 Oct;12(10):980-982.)
• nutritional support: Folate and possibly vitamin D supplementation is recommended for individuals taking
colestipol.
nutrient affected by drug: Calcium
• mechanism: Colestipol lowers vitamin D absorption and hence adversely effects calcium metabolism. Some research indicates that colestipol can bind calcium and thereby further decrease absorption.
(Leonard JP, et al. Arzneimittelforschung 1979;29(7):979-981.)
nutrient affected by drug: Iron
• mechanism: An iron deficiency can result from long-term use of colestipol.
(Leonard JP, et al. Arzneimittelforschung 1979;29(7):979-981; Schlierf G, et al.
Klin Wochenschr. 1985 Sep 2;63(17):802-806.)
• nutritional support: Individuals taking colestipol would most likely benefit from taking a high-potency multivitamin/mineral supplement to compensate for these interactions.
nutritional concern: Constipation
• mechanism: Bile acid sequestrants, such as colestipol, often cause adverse effects such as abdominal bloating and may produce or worsen pre-existing constipation. Constipation may aggravate hemorrhoids.
• research: Research by Spence et al suggests that adding psyllium to half the usual dose of bile acid sequestrant resins maintains the efficacy and improves the tolerability of these resins.
(Spence JD, et al. Ann Intern Med 1995 Oct 1;123(7):493-499.)
• nutritional support: Individuals taking colestipol would most likely benefit from increased fluid and fiber intake alleviate the constipation. Psyllium seed husk could be particularly beneficial, but only with proportionately increased water intake.

![]()
Please read the disclaimer concerning the intent and limitations of the information provided here.
Do not rely solely on the information in this article.
The information presented in Interactions is for informational and educational purposes only. It is based on scientific studies (human, animal, or in vitro), clinical experience, case reports, and/or traditional usage with sources as cited in each topic. The results reported may not necessarily occur in all individuals and different individuals with the same medical conditions with the same symptoms will often require differing treatments. For many of the conditions discussed, treatment with conventional medical therapies, including prescription drugs or over-the-counter medications, is also available. Consult your physician, an appropriately trained healthcare practitioner, and/or pharmacist for any health concern or medical problem before using any herbal products or nutritional supplements or before making any changes in prescribed medications and/or before attempting to independently treat a medical condition using supplements, herbs, remedies, or other forms of self-care.
References
Azen SP, Qian D, Mack WJ, Sevanian A, Selzer RH, Liu CR, Liu CH, Hodis HN. Effect of supplementary antioxidant vitamin intake on carotid arterial wall intima-media thickness in a controlled clinical trial of cholesterol lowering.
Circulation 1996 Nov 15;94(10):2369-2372.
Abstract: BACKGROUND: There is accumulating experimental, epidemiological, and clinical evidence of an association between anti-oxidant vitamin intake and reduced risk of coronary heart disease. Using data from the Cholesterol Lowering Atherosclerosis Study (CLAS), we explored the association of self-selected supplementary antioxidant vitamin intake on the rate of progression of early preintrusive atherosclerosis. METHODS AND RESULTS: CLAS was an arterial imaging trial in which nonsmoking 40- to 59-year-old men with previous coronary artery bypass graft surgery were randomized to colestipol/niacin plus diet or placebo plus diet. The rate of progression of early preintrusive atherosclerosis was determined in 146 subjects using high-resolution B-mode ultrasound quantification of the distal common carotid artery far wall intima-media thickness (IMT). From the nutritional supplement database, 22 subjects had an on-trial average supplementary vitamin E intake of > or = 100 IU per day (high users) and 29 subjects had an average on-trial supplementary vitamin C intake of > or = 250 mg per day (high users). Within the placebo group, less carotid IMT progression was found for high supplementary vitamin E users when compared with low vitamin E users (0.008 versus 0.023 mm/y, P = .03). No effect of vitamin E within the drug group was found. No effect of vitamin C within the drug or placebo group was found. CONCLUSIONS: Supplementary vitamin E intake appears to be effective in reducing the progression of atherosclerosis in subjects not treated with lipid-lowering drugs while the process is still confined to the arterial wall (early preintrusive atherosclerosis).
Bays HE, Dujovne CA. Drug interactions of lipid-altering drugs. Drug Saf 1998 Nov;19(5):355-371. (Review)
Farmer JA, Gotto AM Jr. Antihyperlipidaemic agents. Drug interactions of clinical significance.
Drug Saf 1994 Nov;11(5):301-309.
Abstract: The available antihyperlipidaemic drugs are generally safe and effective, and major systemic adverse effects are uncommon. However, because of their complex mechanisms of action, careful monitoring is required to identify and correct potential drug interactions. Bile acid sequestrants are the most difficult of these agents to administer concomitantly, because their nonspecific binding results in decreased bioavailability of a number of other drugs, including thiazide diuretics, digitalis preparations, beta-blockers, coumarin anticoagulants, thyroid hormones, fibric acid derivatives and certain oral antihyperglycaemia agents. Although the incidence is low, nicotinic acid may cause hepatic necrosis and so should not be used with drugs that adversely affect hepatic structure or function. With the HMG-CoA reductase inhibitors, relatively new agents for which clinical data are still being accumulated, the major problems appears to be rhabdomyolysis, associated with the concomitant use of cyclosporin, fibric acid derivatives or erythromycin, and mild, intermittent hepatic abnormalities that may be potentiated by other hepatotoxic drugs. Fibrates also have the potential to cause rhabdomyolysis, although generally only in combination with HMG-CoA reductase inhibitors, and are subject to binding by concomitantly administered bile acid sequestrants. The major interaction involving probucol is a possible additive effect with drugs or clinical conditions that alter the prolongation of the QTc interval, increasing the potential for polymorphic ventricular tachycardia.
Hodis HN, Mack WJ, LaBree L, Cashin-Hemphill L, Sevanian A, Johnson R, Azen SP. Serial coronary angiographic evidence that antioxidant vitamin intake reduces progression of coronary artery atherosclerosis.
JAMA 1995 Jun 21;273(23):1849-1854.
Abstract: OBJECTIVE--To explore the association of supplementary and dietary vitamin E and C intake with the progression of coronary artery disease. DESIGN--A subgroup analysis of the on-trial antioxidant vitamin intake database acquired in the Cholesterol Lowering Atherosclerosis Study, a randomized, placebo-controlled, serial angiographic clinical trial evaluating the risk and benefit of colestipol-niacin on coronary artery disease progression. SETTING--Community- and university-based cardiac catheterization laboratories. SUBJECTS--A total of 156 men aged 40 to 59 years with previous coronary artery bypass graft surgery. INTERVENTION--Supplementary and dietary vitamin E and C intake (nonrandomized) in association with cholesterol-lowering diet and either colestipol-niacin or placebo (randomized). OUTCOME--Change per subject in the percentage of vessel diameter obstructed because of stenosis (%S) determined by quantitative coronary angiography after 2 years of randomized therapy on all lesions, mild/moderate lesions (< 50%S), and severe lesions (> or = 50%S). RESULTS--Overall, subjects with supplementary vitamin E intake of 100 IU per day or greater demonstrated less coronary artery lesion progression than did subjects with supplementary vitamin E intake less than 100 IU per day for all lesions (P = .04) and for mild/moderate lesions (P = .01). Within the drug group, benefit of supplementary vitamin E intake was found for all lesions (P = .02) and mild/moderate lesions (P = .01). Within the placebo group, benefit of supplementary vitamin E intake was not found. No benefit was found for use of supplementary vitamin C exclusively or in conjunction with supplementary vitamin E, use of multivitamins, or increased dietary intake of vitamin E or vitamin C. CONCLUSIONS--These results indicate an association between supplementary vitamin E intake and angiographically demonstrated reduction in coronary artery lesion progression. Verification from carefully designed, randomized, serial arterial imaging end point trials is needed.
Knodel LC, Talbert RL. Adverse effects of hypolipidaemic drugs. Med Toxicol
1987 Jan;2(1):10-32.
Abstract: Cholestyramine, colestipol, clofibrate, gemfibrozil, nicotinic acid (niacin), probucol, neomycin, and dextrothyroxine are the most commonly used drugs in the treatment of hyperlipoproteinaemic disorders. While adverse reaction data are available for all of them, definitive data regarding the frequency and severity of potential adverse effects from well-controlled trials using large numbers of patients (greater than 1000) are available only for cholestyramine, clofibrate, nicotinic acid and dextrothyroxine. In adult patients treated with cholestyramine, gastrointestinal complaints, especially constipation, abdominal pain and unpalatability are most frequently observed. Continued administration along with dietary manipulation (e.g. addition of dietary fibre) and/or stool softeners results in diminished complaints during long term therapy. Large doses of cholestyramine (greater than 32 g/day) may be associated with malabsorption of fat-soluble vitamins. Most significantly, osteomalacia and, on rare occasions, haemorrhagic diathesis are reported with cholestyramine impairment of vitamin D and vitamin K absorption, respectively. Paediatric patients have been reported to experience hyperchloraemic metabolic acidosis or gastrointestinal obstruction. Concurrent administration of acidic drugs may result in their reduced bioavailability. Serious adverse reactions to clofibrate will probably limit its role in the future. Of particular concern are ventricular arrhythmias, induction of cholelithiasis and cholecystitis, and the potential for promoting gastrointestinal malignancy which far outweigh the reported benefits in preventing new or recurrent myocardial infarction, cardiovascular death and overall death. Patients with renal disease are particularly prone to myositis, secondary to alterations in protein binding and impaired renal excretion of clofibrate. Drug interactions with coumarin anticoagulants and sulphonylurea compounds may produce bleeding episodes and hypoglycaemia, respectively. Nicotinic acid produces frequent adverse effects, but they are usually not serious, tend to decrease with time, and can be managed easily. Dermal and gastrointestinal reactions are most common. Truncal and facial flushing are reported in 90 to 100% of treated patients in large clinical trials. Significant elevations of liver enzymes, serum glucose, and serum uric acid are occasionally seen with nicotinic acid therapy. Liver enzyme elevations are more common in patients given large dosage increases over short periods of time, and in patients treated with sustained release formulations.
Leonard JP, Desager JP, Beckers C, Harvengt C. In vitro binding of various biological substances by two hypocholesterolaemic resins. Cholestyramine and colestipol.
Arzneimittelforschung 1979;29(7):979-981.
Abstract: The ability of cholestyramine and colestipol, two hypocholesterolaemic resins, to bind in vitro several compounds such as vitamin B12, vitamin B12-intrinsic factor complex, folic acid, iron citrate and calcium chloride was investigated. Both resins bound to a high extent vitamin B12-intrinsic factor complex, folic acid and iron citrate; in addition, cholestyramine also caused appreciable binding of calcium. Throughout a large range of pH, there was no change in the binding capacity; however, at pH 2, cholestyramine exhibited a marked drop in the binding of tested substances (with exception of folic acid). By increasing the molarity of the solutions, the binding to the resins of vitamin B12-intrinsic factor complex and of calcium chloride was completely inhibited. In human gastric and duodenal juices, the uptake by the resins of the studied compounds depends on the molarity of the physiological medium tested and partly confirms the results obtained with aqueous solutions. These data obtained in vitro emphasize the necessity of regular monitoring these biochemical parameters during chronic treatment of hypercholesterolaemia conducted with these two resins.
Probstfield JL, Lin TL, Peters J, Hunninghake DB. Carotenoids and vitamin A: the effect of hypocholesterolemic agents on serum levels.
Metabolism 1985 Jan;34(1):88-91.
Abstract: Serum total carotenoid (STC) and vitamin A levels were done as part of the biochemical screening in comparative studies of lipid lowering agents in type Ila hyperlipoproteinemic patients. STC levels were reduced following bile acid sequestering agent administration (colestipol 30 g/d) by 30% (P less than 0.01). Clofibrate and avicel placebo had inconsistent and nonsignificant effects on the STC levels. Serum vitamin A levels were not significantly altered by any of the test agents. The STC level changes were not correlated with concomitant changes in low-density lipoprotein-cholesterol (LDL-C) during any of the treatment regimens. It is suggested that STC level changes are related to alterations in the absorption of carotenoids during bile acid sequestrant administration.
Roe DA. Drug and nutrient interactions in the elderly diabetic. Drug Nutr Interact. 1988;5(4):195-203. (Review)
Schlierf G, Vogel G, Kohlmeier M, Vuilleumier JP, Huppe R, Schmidt-Gayk H. [Long-term therapy of familial hypercholesterolemia in young patients with colestipol: availability of minerals and vitamins].
Klin Wochenschr. 1985 Sep 2;63(17):8020-806. [Article in German]
Abstract: Longterm treatment for 5 years of young patients with familial hypercholesterolemia was accompanied by monitoring of plasma levels of calcium, iron, sodium, parathyroid hormone and water- and fat soluble vitamins, since interference of the ion exchange resin with absorption of numerous substances as well as abnormal plasma levels of some of the above had been described in several studies. Treatment was effective in the group with good compliance (lowering of plasma cholesterol at the end of 5 years by 19% and, compared to the control group, by 23%). Adverse drug effects with respect to the above parameters were not found. Plasma levels of carotinoides and vitamin E were elevated in the patients according to elevated concentrations of lipoproteins which are carriers of these vitamins.
Schwarz KB, Goldstein PD, Witztum JL, Schonfeld G. Fat-soluble vitamin concentrations in hypercholesterolemic children treated with
colestipol. Pediatrics. 1980 Feb;65(2):243-250.
Abstract: In summary: (1) Colestipol therapy plus diet reduced total cholesterol 19 +/- 3% in 11 hypercholesterolemic children after two months and 13 +/- 5% after two years in five children. (2) Diet therapy did not significantly reduce serum concentration of any of the fat-soluble vitamins or folate. (3) During 24 months of colestipol therapy plus diet serum vitamin A and E concentrations did decrease in the five patients with good drug adherence (vitamin A, 68 +/- 11 vs 35 +/- 4 microgram/100 ml, P less than .005) (vitamin E, 14 +/- 1 vs 11 +/- 1 microgram/ml, P less than .05). However, those concentrations remained within normal limits. (4) Colestipol therapy plus diet had no effect on prothrombin time, serum 25-hydroxycholecalciferol, folate, or calcium metabolism (as studied by sequential determination of serum total and ionized calcium phosphorus, alkaline phosphatase, and parathyroid hormone).
Spence JD, Huff MW, Heidenheim P, Viswanatha A, Munoz C, Lindsay R, Wolfe B, Mills D. Combination therapy with colestipol and psyllium mucilloid in patients with
hyperlipidemia. Ann Intern Med 1995 Oct 1;123(7):493-499.
Abstract: OBJECTIVE: To test whether combining psyllium mucilloid with half the usual dose of colestipol reduces the adverse effects associated with colestipol and maintains or increases its efficacy in the treatment of hyperlipidemia. This strategy might make bile acid sequestrants, which are seldom used because they cause adverse effects such as bloating and constipation, more tolerable and less expensive. DESIGN: A randomized, parallel-group, double-blind, controlled trial. SETTING: An outpatient clinic in a tertiary care hospital. PATIENTS: 121 patients who had moderate primary hypercholesterolemia (total cholesterol level > 6 mmol/L and < 8 mmol/L; triglyceride level < 3 mmol/L) after following a low-fat diet for 1 year (National Cholesterol Education Program Step Two diet). INTERVENTION: 5 g of cellulose placebo; 5 g of colestipol; 2.5 g of colestipol plus 2.5 g of psyllium; or 5 g of psyllium three times daily before meals for 10 weeks. MAIN OUTCOME MEASURES: At baseline and at weeks 4 and 10, fasting blood lipid levels and apoprotein concentrations were measured and a quality-of-life instrument was completed. RESULTS: A combination of 2.5 g of psyllium and 2.5 g of colestipol was better tolerated than and as effective as either 5 g of colestipol alone or 5 g of psyllium alone. The combination therapy and colestipol alone did not differ significantly with respect to changes in individual lipid values. The ratio of total cholesterol to high-density lipoprotein cholesterol (HDL) was reduced by 18.2% (95% CI, 12.3% to 24%) with the combination therapy; by 10.6% (CI, 2.0% to 15.4%) with colestipol alone; by 6.1% (CI, 1.5% to 10.6%) with psyllium alone; and by 0.1% (CI, -4.8% to 7%) with placebo (P = 0.0002). Combination therapy reduced the ratio of total cholesterol to HDL significantly more than did colestipol alone or psyllium alone (P < 0.05). CONCLUSIONS: These findings suggest that adding psyllium to half the usual dose of bile acid sequestrant resins maintains the efficacy and improves the tolerability of these resins.
Tonstad S, Sivertsen M, Aksnes L, Ose L. Low dose colestipol in adolescents with familial
hypercholesterolaemia. Arch Dis Child 1996 Feb;74(2):157-160.
Abstract: The effects of orange flavoured colestipol granules, 10 g/day, in 37 boys and 29 girls aged 10-16 years with familial hypercholesterolaemia were examined first in an eight week double blind, placebo controlled protocol, then in open treatment for 44-52 weeks. All patients were on a low fat diet. Low density lipoprotein cholesterol levels were reduced by 19.5% by colestipol v 1.0% by placebo. Levels of serum folate, vitamin E, and carotenoids were reduced in the colestipol group, but not the vitamin E/cholesterol and carotenoid/cholesterol ratios or serum concentrations of vitamins A and D. After one year of colestipol, two thirds of the participants remained in the study, of whom half took > or = 80% of the prescribed dose. Those who took > or = 80% of the dose had a greater decrease in serum 25-hydroxyvitamin D levels than those who took < 80%. No adverse effects on weight gain or linear growth velocity were observed. Although low dose colestipol effectively reduces low density lipoprotein cholesterol levels, only a minority of adolescents adhered to the new formulation for one year. Folate and possibly vitamin D supplementation is recommended.
Tsang RC, Roginsky MS, Mellies MJ, Glueck CJ. Plasma 25-hydroxy-vitamin D in familial hypercholesterolemic children receiving colestipol resin.
Pediatr Res. 1978 Oct;12(10):980-982.
Werbach, MR. Foundations of Nutritional Medicine. Tarzana, CA: Third Line Press, 224, 1997. (Review)