
Calcium
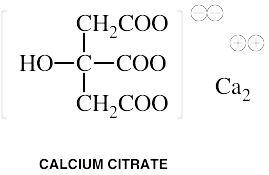
Common Names: Calcium ascorbate, Calcium aspartate, Calcium carbonate, Calcium citrate, Calcium lactate, Microcrystalline hydroxyapatite (MCHC)Please read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
[No author given.] Drug Evaluations Subscription. Chicago: American Medical Association, Vol. I, Section 7. Chapter 2, Spring, 1992.
[No author given.] Drug Evaluations Subscription. Chicago, IL: American Medical Association, Vol. II, Section 10, Chapter 2, Summer, 1993.
Aarskog D, Aksnes L, Lehmann V. Low 1,25-dihydroxyvitamin D in heparin-induced osteopenia.
Lancet 1980 Sep 20;2(8195 Pt 1):650-651. (Letter)
Aarskog D, Aksnes L, Markestad T, Ulstein M, Sagen N. Heparin-induced inhibition of 1,25-dihydroxyvitamin D formation.
Am J Obstet Gynecol 1984 Apr 15;148(8):1141-1142.
Adlin EV, Maurer AH, Marks AD, Channick BJ. Bone mineral density in postmenopausal women treated with L-thyroxine.
Am J Med 1991 Mar;90(3):360-366.
Abstract: PURPOSE: To determine if bone mineral density is decreased in postmenopausal women treated with 1-thyroxine, and, if any decrease is observed, whether it is related to overtreatment with thyroid hormone, to deficiency of calcitonin, or to other factors. PATIENTS AND METHODS: The study consisted of 19 postmenopausal women between 50 and 75 years of age treated with 1-thyroxine for 5 years or longer, and 19 matching control subjects with no thyroid disease. Bone mineral density of the spine and hip was measured by dual-photon absorptiometry. Plasma calcitonin concentrations and serum thyroid hormone levels were determined by radioimmunoassays. RESULTS: The 1-thyroxine-treated women had lower bone density in the lumbar spine (1.013 g/cm2 [95% confidence interval, 0.945 to 1.081] versus 1.134 g/cm2 [1.026 to 1.242], p = 0.043); in the femoral neck (0.736 g/cm2 [0.694 to 0.778] versus 0.809 g/cm2 [0.747 to 0.872], p = 0.040); in Ward's triangle (0.576 g/cm2 [0.530 to 0.623] versus 0.694 g/cm2 [0.617 to 0.770], p = 0.011); and in the trochanteric area (0.626 g/cm2 [0.581 to 0.672] versus 0.722 g/cm2 [0.651 to 0.794], p = 0.027). The maximal increase in calcitonin following calcium infusion was 1.37 ng/L (95% confidence interval, -0.44 to 3.17) in the 1-thyroxine-treated patients versus 18.8 ng/L (95% confidence interval, 10.0 to 27.5) in normal women, p less than 0.001. The average dose of 1-thyroxine was 120 micrograms/day; 16 of the 19 patients had normal serum thyroxine levels. However, TSH levels were low in 13 of the 19, suggesting that 1-thyroxine treatment was supraphysiologic. Seven of the 19 patients had a history of hyperthyroidism in the distant past; these patients, considered separately, had significantly reduced bone density in the hip. The other 12 patients, considered separately, did not have a statistically significant loss of bone density. CONCLUSIONS: Long-term 1-thyroxine therapy is associated with decreased density of the spine and hip. Since subclinical hyperthyroidism, decreased calcitonin responsiveness, and a history of hyperthyroidism were demonstrated in some or all of these patients, these factors must be considered as possible causes of the decreased bone density.
Bar-Or D, Gasiel Y. Calcium and calciferol antagonise effect of verapamil in atrial fibrillation.
Br Med J (Clin Res Ed) 1981 May 16;282(6276):1585-1586.
Bianchetti MG, Kanaka C, Ridolfi-Luthy A, Wagner HP, Hirt A, Paunier L, Peheim E, Oetliker OH. Chronic renal magnesium loss, hypocalciuria and mild hypokalaemic metabolic alkalosis after cisplatin.
Pediatr Nephrol 1990 May;4(3):219-222.
Abstract: Renotubular handling of sodium, potassium (K) calcium (Ca), phosphate, hydrogen ions and glucose, and urinary concentrating ability were studied in three children (aged 8, 8.5, 11 years) with renal magnesium (Mg) loss, persisting for more than 2 years after discontinuation of cisplatin treatment for neuroblastoma. A group of healthy children served as controls. Besides renal Mg wasting, a clear-cut tendency towards reduced calciuria associated with normal or slightly elevated plasma Ca was observed. Plasma K tended to be low (3.4-3.7 mmol/l), and plasma chloride was normal. Plasma bicarbonate (HCO3) ranged from 24.9 to 27.8 mmol/l, and urinary pH was always less than 6.0, indicating a renal HCO3 threshold exceeding 24 mmol/l. Plasma creatinine levels, glucosuria and phosphaturia, and urinary concentrating capacity were adequate. Comparable features were found in three children (aged 4.5, 9, 13 years) with primary renotubular hypomagnesaemia-hypokalaemia and hypocalciuria. This study complements the picture of chronic cisplatin tubulopathy in childhood demonstrating that, apart from Mg wasting, a reduced Ca excretion, and a tendency to hypokalaemia and metabolic alkalosis exist. Thus cisplatin may induce renal functional damage identical to that found in primary renotubular hypomagnesaemia--hypokalaemia with hypocalciuria.
Bolsin S, Jones S. Acute renal failure potentiated by gentamicin and calcium.
Anaesth Intensive Care 1997 Aug;25(4):431-432. (Letter)
Bourgoin BP, Evans DR, Cornett JR, et al. Lead content in 70 brands of dietary calcium supplements.
Am J Publ Health 1993;83:1155-1160.
Brouwers JR. Drug interactions with quinolone antibacterials. Drug Saf 1992 Jul-Aug;7(4):268-281. (Review)
Abstract: The quinolone antibacterials are prone to many interactions with other drugs. Quinolone absorption is markedly reduced with antacids containing aluminium, magnesium and/or calcium and therapeutic failure may result. Other metallic ion-containing drugs, such as sucralfate, iron salts, and zinc salts, can also reduce absorption. Some of the newer quinolones inhibit the cytochrome P450 system, e.g. enoxacin, pefloxacin and ciprofloxacin. The toxicity of drugs that are metabolised by the cytochrome P450 system is enhanced by concomitant use of some quinolones. Ciprofloxacin, enoxacin and pefloxacin can increase theophylline concentrations to toxic values. The pharmacokinetics of warfarin and cyclosporin are unaffected. Ofloxacin, fleroxacin and temafloxacin have a low inhibitory effect on the cytochrome P450 system and a low interaction potential may result. The affinity of quinolones for the gamma-aminobutyric acid (GABA) receptor may induce CNS adverse effects; these effects are enhanced by some nonsteroidal anti-inflammatory drugs (NSAIDs).
Buckley LM, Leib ES, Cartularo KS, Vacek PM, Cooper SM. Calcium and vitamin D3 supplementation prevents bone loss in the spine secondary to low-dose corticosteroids in patients with rheumatoid arthritis. A randomized, double-blind, placebo-controlled trial.
Ann Intern Med 1996 Dec 15;125(12):961-968.
Abstract: BACKGROUND: Therapy with low-dose corticosteroids is commonly used to treat allergic and autoimmune diseases. Long-term use of corticosteroids can lead to loss of bone mineral density and higher risk for vertebral fractures. Calcium and vitamin D3 supplementation is rational therapy for minimizing bone loss, but little evidence for its effectiveness exists. OBJECTIVE: To assess 1) the effects of supplemental calcium and vitamin D3 on bone mineral density of patients with rheumatoid arthritis and 2) the relation between the effects of this supplementation and corticosteroid use. DESIGN: 2-year randomized, double-blind, placebo-controlled trial. SETTING: University outpatient-care facility. PATIENTS: 96 patients with rheumatoid arthritis, 65 of whom were receiving treatment with corticosteroids (mean dosage, 5.6 mg/d). INTERVENTION: Calcium carbonate (1000 mg/d) and vitamin D3 (500 IU/d) or placebo. MEASUREMENTS: Bone mineral densities of the lumbar spine and femur were determined annually. RESULTS: Patients receiving prednisone therapy who were given placebo lost bone mineral density in the lumbar spine and trochanter at a rate of 2.0% and 0.9% per year, respectively. Patients receiving prednisone therapy who were given calcium and vitamin D3 gained bone mineral density in the lumbar spine and trochanter at a rate of 0.72% (P = 0.005) and 0.85% (P = 0.024) per year, respectively. In patients receiving prednisone therapy, bone mineral densities of the femoral neck and the Ward triangle did not increase significantly with calcium and vitamin D3. Calcium and vitamin D3 did not improve bone mineral density at any site in patients who were not receiving corticosteroids. CONCLUSION: Calcium and vitamin D3 prevented loss of bone mineral density in the lumbar spine and trochanter in patients with rheumatoid arthritis who were treated with low-dose corticosteroids.
Buist RA. Drug-nutrient interactions - an overview. Intl Clin Nutr Rev 1984;4(3):114. (Review)
Burros M. Testing calcium supplements for lead. New York Times June 4,1997, B7.
Camici M. Dialysis-associated bone disease. JAMA 1986 Sep 19;256(11):1447. (Letter)
Chesney RW, Mazess RB, Hamstra AJ, DeLuca HF, O'Reagan S. Reduction of serum-1,25-dihydroxyvitamin-D, in children receiving glucocorticoids.
Lancet 1978 Nov 25;2(8100):1123-1125.
Abstract: Serum-1,25-dihydroxyvitamin-D3 (1,25-[OH]2D3) was subnormal in children receiving long-term glucocorticoid treatment for various glomerular diseases, including nephrotic syndrome. In children with chronic glomerulonephritis not treated with glucocorticoids who had similar serum-creatinine with glucocorticoids who had similar serum-creatinine concentrations, serum-1,25-dihydroxyvitamin-D3 concentrations resembled those in healthy controls, indicating that glomerular renal disease per se does not account for reduced serum-1,25(OH)2DE concentrations in steroid-treated patients. The reduction in concentration of this most active vitamin-D metabolite correlated with the dose of steroid administered and with reduction in forearm bone mineral content measured by the photon absorption technique. Reduced serum-1,25-(OH)2D3 concentration may be important in the pathogenesis of steroid-induced osteopenia.
Christiansen C, Rodbro P, Lund M. Incidence of anticonvulsant osteomalacia and effect of vitamin D: controlled therapeutic trial.
Br Med J 1973 Dec 22;4(894):695-701
Chung S, Ahn C. Effects of anti-epileptic drug therapy on bone mineral density in ambulatory epileptic children.
Brain Dev 1994 Sep-Oct;16(5):382-385.
Abstract: In order to assess the bone changes in the subjects receiving anti-epileptic drugs (AEDs), bone mineral densities (BMDs) of the arms, legs, ribs, pelvis, spine, and the whole body were scanned in 78 epileptic children and in 78 controls using dual photon absorptiometry. The study subjects were classified according to the duration of the monotherapy with phenobarbital (PB) or phenytoin (PHT); those who received AEDs for less than 12 months as Group I, for 13-23 months as Group II, and for 24 months as Group III. Group III was subclassified according to the kind of AEDs administered, into those receiving PB as Group IIIp, and those receiving PHT as Group IIId. There was no significant differences in the BMDs of each area, when compared to each control in Groups I and II. In Group III, there were significant differences in ribs and spine, according to the duration of administration. In Group IIIp, there was a significant difference in ribs and spine, and, in Group IIId, there was a significant difference in most of the areas. These results show that the measurement of BMDs in the ribs and spine is necessary for the early detection of subtle bone loss, and it is recommended that vitamin D be administered to children with epilepsy receiving AEDs over 24 months.
Coburn JW, Mischel MG, Goodman WG, Salusky IB. Calcium citrate markedly enhances aluminum absorption from aluminum hydroxide.
Am J Kidney Dis. 1991 Jun;17(6):708-711.
Abstract: The effect of calcium citrate on intestinal aluminum absorption, assessed by the increment in urinary aluminum excretion, was evaluated in eight normal men. Baseline urinary aluminum excretion was determined for 2 days; thereafter, subjects ingested aluminum hydroxide for 3 days. In a cross-over study, subjects were given either calcium citrate, 950 mg four times a day, or placebo during the 3 days of aluminum hydroxide ingestion (2.4 g/d). Plasma aluminum levels were measured on the second control day and the third day of aluminum hydroxide ingestion. Baseline urinary aluminum excretion was 0.02 +/- 0.004 (6.5 +/- 1.1 micrograms/g creatinine) and 0.03 +/- 0.005 mumol/mmol creatinine (7.4 +/- 1.3 micrograms/g creatinine). These values increased during aluminum hydroxide therapy, but values were much greater when calcium citrate was ingested with aluminum hydroxide. On 3 consecutive days, urinary aluminum excretion levels were 11.1 +/- 3.23, 8.8 +/- 2.9, and 5.3 +/- 0.7 times greater during the administration of calcium citrate with aluminum hydroxide than with aluminum hydroxide alone. Plasma aluminum levels did not differ in the two treatment groups. Thus, calcium citrate markedly enhances the absorption of aluminum from aluminum hydroxide and the two must not be prescribed together in patients with renal failure.
Collins N, Maher J, Cole M, Baker M, Callaghan N. A prospective study to evaluate the dose of vitamin D required to correct low 25-hydroxyvitamin D levels, calcium, and alkaline phosphatase in patients at risk of developing antiepileptic drug-induced osteomalacia. Q J Med 1991 Feb;78(286):113-122.
Abstract: The dose of vitamin D3 required to maintain normal serum 25-hydroxyvitamin D levels in epileptic patients was evaluated in a prospective study. Patients were divided into two groups, comprising 14 institutionalized and 18 non-institutionalized subjects; they were taking carbamazepine, phenytoin and phenobarbitone, alone or in combination. The study was divided into a dose titration stage and a further period of assessment on a fixed dose after attainment of normal serum 25-hydroxyvitamin D levels. Seventeen of the 18 non-institutionalized patients achieved normal levels over a period of 12 months; the remaining patient became normal after 15 months. The dose required to achieve normal levels ranged from 400 to 4000 IU/day; three patients required less than 2400 IU vitamin D3, 12 required 2400 IU and three required greater than 2400 IU. All institutionalized patients achieved normal levels over a period of 12 months; six patients required less than 2400 IU, six required 2400 IU and two required greater than 2400 IU vitamin D3. Raised alkaline phosphatase levels occurred in 11 patients, and reverted to normal in six patients during the initial return of 25-hydroxyvitamin D levels to normal. During the second 12 months, when patients were taking a fixed dose of vitamin D3, alkaline phosphatase increased in five patients who had achieved normal levels. During this phase normal 25-hydroxyvitamin D levels were not maintained in five patients. There was a significant seasonal variation of 25-hydroxyvitamin D levels institutionalized patients, being highest in June and lowest in December. Our findings show that while there was a wide range in the dose required to achieve normal serum 25-hydroxyvitamin D levels--between 400 and 4000 IU/day--78 per cent of patients responded to a dose of 2400 IU/day.
Dahlman T, Lindvall N, Hellgren M. Osteopenia in pregnancy during long-term heparin treatment: a radiological study post partum.
Br J Obstet Gynaecol. 1990 Mar;97(3):221-228.
Abstract: Osteopenia, sometimes with compression fractures of the spine, is a side-effect of long-term heparin treatment. The frequency is unknown. In this study, 70 women were given subcutaneous heparin as therapy for, or prophylaxis against, thromboembolism during pregnancy. All, except two, were examined by X-ray of the spine and hip first week post partum. The duration of treatment and the dosage of heparin varied. There were 12 (17%) with obvious osteopenia, including two women with multiple fractures of the spine (3%). Re-examination 6-12 months post partum showed that the changes were reversible in most cases. Another 18 women were examined about three years after heparin treatment during pregnancy. No obvious osteopenia was found among them or in a control group of 30 women examined in the first week post partum. The degree of osteopenia was not correlated with either the heparin dose or the duration of treatment. Women treated with heparin in consecutive pregnancies do not seem to have an increased risk of osteopenia.
D'Arcy PF, Griffin, JP. Iatrogenic Diseases. London: Oxford U. Press, 1972.
D'Erasmo E, Ragno A, Raejntroph N, Pisani D. [Drug-induced osteomalacia]. Recenti Prog Med
1998 Oct;89(10):529-533. [Article in Italian] (Review)
Abstract: The osteomalacia is a metabolic bone disease, characterized by a defect of bone mineralization, due to a lot of causes; among these an important role may be attributed to some drugs. The drugs most frequently associated with osteomalacia are: cholestyramine, phenytoin, phenobarbital, rifampicin, isoniazid, aluminium-containing antacid, saccharated ferric oxide, cadmium, lead, bisphosphonates, fluoride and aluminum. In this review we discuss about the pathophysiologic mechanisms related to drug-induced osteomalacia involving vitamin D metabolism, phosphorus homeostasis and bone mineralization.
Duus BR Fractures caused by epileptic seizures and epileptic osteomalacia. Injury 1986 Jan;17(1):31-33.
Abstract: A case of several severe fractures in one patient following epileptic seizures is reported. The patient suffered from epileptic osteomalacia and responded well to vitamin D treatment. The cause of anticonvulsant-induced osteomalacia and its treatment are discussed.
Elliott WC, Patchin DS. Effects and interactions of gentamicin, polyaspartic acid and diuretics on urine calcium concentration.
J Pharmacol Exp Ther 1995 Apr;273(1):280-284.
Abstract: Gentamicin causes isolated, reversible calciuria in rats by an unknown mechanism. We hypothesized that gentamicin calciuria is related to nonantibacterial properties that may interfere with transtubular calcium transport (calcium channel blockade, Na,K-ATPase inhibition or competition with calcium for binding to the brush-border membrane). The calciuric effect of gentamicin was compared to the calcium channel blockers lanthanum and cobalt, the Na,K-ATPase inhibitor ouabain and the polycation aprotinin (which competes with gentamicin for brush-border membrane binding). Although gentamicin 0.02 mmol/kg caused a 6-8-fold increase in urine calcium concentration, none of the other agents was calciuric. We also found that the calciuric effects of gentamicin and furosemide were additive, whereas the noncalciuric diuretic chlorothiazide had no effect on gentamicin calciuria. We also determined the effect of poly-L-aspartic acid (PAA), which binds gentamicin and prevents nephrotoxicity. PAA caused isolated calciuria similar in magnitude and character to gentamicin. However, PAA pretreatment decreased the magnitude of gentamicin calciuria to insignificance. PAA pretreatment did not prevent furosemide calciuresis. These results indicate that: 1) gentamicin and furosemide calciuria are caused by different mechanisms; 2) gentamicin calciuria is probably not mediated by calcium channel blockade, Na,K-ATPase inhibition or displacement of brush-border membrane-bound calcium; 3) gentamicin and PAA calciuria may reflect interference with intracellular events related to transtubular calcium transport.
Fox J, Della-Santina CP. Oral verapamil and calcium and vitamin D metabolism in rats: effect of dietary calcium.
Am J Physiol 1989 Nov;257(5 Pt 1):E632-638.
Franklyn J, Betteridge J, Holder R, Daykin J, Lilley J, Sheppard M. Bone mineral density in thyroxine treated females with or without a previous history of thyrotoxicosis.
Clin Endocrinol (Oxf) 1994 Oct;41(4):425-432.
Thyroxine therapy alone does not represent a significant risk factor for loss of bone mineral density but there is a risk of bone loss in post-menopausal (but not premenopausal) females with a previous history of thyrotoxicosis treated with radioiodine.
Abstract: OBJECTIVE: The results of studies examining the influence of T4 therapy upon bone mineral density (BMD) are conflicting. This conflict may, in part, reflect inclusion of patients with varying thyroid disorders. We have therefore examined the influence of preceding thyroid history and T4 therapy on BMD. DESIGN: Case-control studies of patients on long-term T4 therapy who have or have not previously received radioiodine treatment for thyrotoxicosis, as well as previously thyrotoxic patients who have not required T4 replacement. PATIENTS: Twenty-seven premenopausal and 60 postmenopausal females with a past history of thyrotoxicosis and subsequent T4 treated hypothyroidism (group 1), 39 post-menopausal females with a past history of radioiodine treated thyrotoxicosis not receiving T4 (group 2) and 22 post-menopausal females with primary hypothyroidism on T4 (group 3). Female controls individually matched to patients by age and menopausal status. MEASUREMENTS: BMD measured by dual-energy X-ray absorptiometry. Serum biochemistry and tests of thyroid function. RESULTS: No significant differences were found in femoral or lumbar spine BMD measurements between premenopausal patients and controls in group 1 or between group 2 patients and controls. Measurements of BMD at all sites were lower in post-menopausal patients in groups 1 and 2 than in controls; when allowance was made for differences in BMD due to body mass index by analysis of variance, significant reductions in femoral trochanter BMD (3.9%, P < 0.05) and lumbar spine (5.6-8.5%, P < 0.01) BMD results were found in post-menopausal females in group 1 and reductions in femoral trochanter (3.9%, P < 0.01), Ward's triangle (5.6%, P < 0.05) and lumbar spine (8.5%, P < 0.01) BMD results in group 2. Separate analysis of BMD results of those with normal or reduced serum TSH did not affect outcome. BMD measurements were not significantly correlated with duration of T4 therapy, T4 dose, or serum free T4 or TSH in any patient group. CONCLUSIONS: Thyroxine therapy alone does not represent a significant risk factor for loss of bone mineral density but there is a risk of bone loss in post-menopausal (but not premenopausal) females with a previous history of thyrotoxicosis treated with radioiodine.
Franklyn JA, Betteridge J, Daykin J, Holder R, Oates GD, Parle JV, Lilley J, Heath DA, Sheppard MC. Long-term thyroxine treatment and bone mineral density.
Lancet 1992 Jul 4;340(8810):9-13.
Abstract: Studies of the effect of thyroxine replacement therapy on bone mineral density have given conflicting results; the reductions in bone mass reported by some have prompted recommendations that prescribed doses of thyroxine should be reduced. We have examined the effect of long-term thyroxine treatment in a large homogeneous group of patients; all had undergone thyroidectomy for differentiated thyroid cancer but had no history of other thyroid disorders. The 49 patients were matched with controls for age, sex, menopausal status, body mass index, smoking history, and calcium intake score; in all subjects bone mineral density at several femoral and vertebral sites was measured by dual-energy X-ray absorptiometry. Despite long-term thyroxine therapy (mean duration 7.9 [range 1-19] years) at doses (mean 191 [SD 50] micrograms/day) that resulted in higher serum thyroxine and lower serum thyrotropin concentrations than in the controls, the patients showed no evidence of lower bone mineral density than the controls at any site. Nor was bone mineral density correlated with dose, duration of therapy, or cumulative intake, or with tests of thyroid function. There was a decrease in bone density with age in both groups. We suggest that thyroxine alone does not have a significant effect on bone mineral density and hence on risk of osteoporotic fractures.
Garland HO, Phipps DJ, Harpur ES. Gentamicin-induced hypercalciuria in the rat: assessment of nephron site involved.
J Pharmacol Exp Ther. 1992 Oct;263(1):293-297.
Abstract: Two independent techniques were used in anesthetized rats in an attempt to locate the nephron site of the reduced tubular calcium reabsorption accompanying acute gentamicin infusion. The first technique was that of lithium clearance used to assess proximal sodium (and secondarily calcium) handling. Observations that lithium clearance was comparable in control and gentamicin-treated animals (1.83 +/- 0.39 vs. 1.46 +/- 0.14 ml.min-1 for first experimental period) suggests a lack of proximal effect of the drug. The second technique was that of tracer microinjection whereby superficial nephrons were injected with 45Ca and tubule calcium transport was assessed from the recovery of radioactivity in the final urine. 45Ca recovery values from distal microinjections were comparable in control and gentamicin-treated groups (81.1 +/- 2.0 vs. 77.7 +/- 4.6%). However, 45Ca recovery values from proximal microinjections were significantly higher in the gentamicin group (9.4 +/- 1.0 vs. 3.5 +/- 0.8%; P < .001). These data suggest that the effects of gentamicin on renal calcium handling are mediated at a nephron site proximal to the distal tubule (i.e., loop of Henle or proximal tubule itself). Closer examination of individual proximal micropuncture data may point to an effect occurring predominantly in the pars recta of the proximal tubule or loop of Henle. Taken together, the results of both parts of the present study suggest that the early physiological effects of gentamicin on the kidney occur in a different nephron segment from any subsequent nephrotoxicity.
Gough H, Goggin T, Bissessar A, Baker M, Crowley M, Callaghan N. A comparative study of the relative influence of different anticonvulsant drugs, UV exposure and diet on vitamin D and calcium metabolism in out-patients with epilepsy.
Q J Med 1986 Jun;59(230):569-577.
Abstract: The biochemical parameters associated with vitamin D metabolism, calcium, 25-hydroxy-vitamin D (25OHD) and alkaline phosphatase levels were assessed in 226 out-patients with epilepsy. Patients were grouped depending on the drug treatment; carbamazepine, phenytoin, phenobarbitone and sodium valproate used alone as monotherapy and a combination of these drugs as polytherapy. The most severe alterations occurred in the polytherapy group. Hypocalcaemia was more severe in the phenobarbitone monotherapy group than the carbamazepine or the phenytoin groups. No patient on sodium valproate monotherapy had subnormal levels of calcium (less than 2.1 mmol/l). 25OHD levels were similarly reduced in the carbamazepine, phenytoin and the phenobarbitone groups with no reduction in the sodium valproate group. Significant elevations in alkaline phosphatase levels were evident in all patient groups except the sodium valproate group. This study confirms biochemical evidence for anticonvulsant osteomalacia when the enzyme-inducing drugs are used, the degree of severity depending on the drug regimen.
Greenspan SL, Greenspan FS, Resnick NM, Block JE, Friedlander AL, Genant HK. Skeletal integrity in premenopausal and postmenopausal women receiving long-term L-thyroxine therapy.
Am J Med 1991 Jul;91(1):5-14.
Abstract: PURPOSE: The impact of long-term L-thyroxine replacement therapy on skeletal integrity is a growing concern because of the large number of women receiving thyroid hormone therapy. The purpose of this study was to examine the hypothesis that long-term L-thyroxine therapy in which the free thyroxine index (FT4I) is maintained within a physiologic range has minimal impact on vertebral or femoral bone mineral density in both premenopausal and postmenopausal women. PATIENTS AND METHODS: We measured hip integral and spinal trabecular and integral bone densities in 28 premenopausal and 28 postmenopausal women who had been receiving L-thyroxine therapy for a median of 12 and 15 years, respectively, and in whom therapy was titrated to keep the FT4I within the normal range. The relationship between bone density parameters and thyroid hormone status was examined using univariate and multivariate statistical methods. RESULTS: Seventy-nine percent of the premenopausal women and 86% of the postmenopausal women had FT4I values in the normal range at the time of bone density determination. Moreover, throughout the study's duration, the majority of annually measured values were in the normal range for more than 80% of subjects. Premenopausal women had slightly lower bone density than would be expected for age: -6.7% (z = -0.39 +/- 0.74 [mean +/- SD], p less than 0.01), -3.1% (z = -0.22 +/- 0.78, p = 0.15), and -5.1% (z = -0.36 +/- 0.74, p less than 0.02) for spinal trabecular, spinal integral, and hip integral bone density, respectively. Postmenopausal women likewise had slightly lower bone density values that were significant only at the hip: -0.2% (z = -0.01 +/- 1.01, p = 0.95), -1.0% (z = -0.05 +/- 1.11, p = 0.80), and -6.2% (z = -0.39 +/- 0.80, p less than 0.02) for spinal trabecular, spinal integral, and hip integral bone density, respectively. When patients with previously treated Graves' disease (n = 4 in each group) were eliminated, the differences in bone density at the hip were no longer seen. Correlation analysis revealed only weak and generally nonsignificant relationships between parameters of thyroid hormone status and bone density at any site in either subgroup. Results of multiple regression analysis among the pooled data of all subjects showed that age provided a consistently significant contribution (R2 = 0.18 to 0.66) to the variability in bone density at the spine and the hip, but parameters of thyroid hormone status did not. CONCLUSION: These data provide the first supportive evidence that long-term L-thyroxine therapy that maintains the FT4I in the physiologic range is associated with a statistically significant, but clinically minimal, decrement in spinal and hip bone density in both premenopausal and postmenopausal women. The decrement at the hip was entirely due to the inclusion of patients with treated Graves' diseases. Thus, the changes in bone density in women receiving long-term L-thyroxine therapy are minimal at most and should not be a contraindication to therapy.
Hammarstrom L. [Side-effects on bone and teeth of the tetracyclines]. Lakartidningen 1968 Jun 4;65:Suppl 2:89-96. [Article in Swedish]
Hanna FW, Pettit RJ, Ammari F, Evans WD, Sandeman D, Lazarus JH. Effect of replacement doses of thyroxine on bone mineral density.
Clin Endocrinol (Oxf) 1998 Feb;48(2):229-234.
Abstract: INTRODUCTION: Hyperthyroidism is associated with a reduction in bone mineral density (BMD). Suppressive doses of thyroxine (T4), inducing subclinical hyperthyroidism, have been reported by some investigators to reduce BMD. Little work has been done on replacement doses of T4. AIM: The aim was to investigate the effect of replacement doses of T4 on BMD. STUDY DESIGN: Cross-sectional study of hypothyroid patients on long-term T4 replacement doses, comparing those who had primary hypothyroidism with those who were previously hyperthyroid. PATIENTS: Fifty women on replacement doses of T4 for more than 5 years were recruited. Twenty-five were treated for primary (group 1) and 25 for radioiodine-induced hypothyroidism (group 2). They were well matched for age, menstrual status, smoking history, body mass index (BMI), dose and duration of T4 replacement as well as thyroid status. MEASUREMENTS: BMD was assessed by dual energy X-ray absorptiometry. Free T4 (FT4), FT3 as well as ultrasensitive TSH assays were used to assess thyroid status. RESULTS: The two groups showed no difference in BMD (g/cm2) of the lumbar spine (1.008 vs. 0.957, P = 0.25), femoral neck (0.745 vs. 0.735, P = 0.79) and total hip (0.878 vs. 0.837, P = 0.24). When the two groups were pooled, there was no significant difference between the patients and a reference population with femoral neck and total hip BMD expressed as a standard deviation (Z) score. However, the lumbar spine mean Z score was significantly greater than zero. For each site, there was a negative correlation of BMD with age in at least one group but, in general, BMI, FT4, FT3 and duration of T4 replacement did not correlate with BMD. T4 dose, however, had a consistent positive correlation with BMD in the spine, femoral neck and the hip (P = 0.01, 0.04 and 0.02, respectively) in group 2 but not group 1. CONCLUSION: In this study, there is no evidence for a difference in bone mineral density in patients receiving replacement doses of thyroxine irrespective of the aetiology of their hypothyroidism. The reduced bone mineral density associated with hyperthyroidism appears to be restored, maintained and in some cases possibly improved while on long-term thyroxine replacement post-radioiodine.
Haram K, Hervig T, Thordarson H, Aksnes L. Osteopenia caused by heparin treatment in pregnancy.
Acta Obstet Gynecol Scand 1993 Nov;72(8):674-675.
Abstract: A case is reported of severe osteopenia caused by heparin treatment of thrombosis in the eleventh week of pregnancy followed by heparin prophylaxis (5000 IU three times daily) during pregnancy and lactation. The mother complained of back pain during the last two weeks of pregnancy. Six weeks post partum, generalized osteopenia in the skeleton was diagnosed and a compression fracture of the body of the sixth thoracic vertebra. During pregnancy the mother had relatively low serum concentrations of 1,25(OH)2D, the active metabolite of vitamin D, and six weeks after delivery the serum concentration had fallen to about 50% of the lowest reference level. Eight and fourteen weeks after delivery, when heparin treatment had been discontinued, the serum concentrations of 1,25(OH)2D were within the reference range for non-pregnant adults.
Hartard M, Bottermann P, Bartenstein P, Jeschke D, Schwaiger M. Effects on bone mineral density of low-dosed oral contraceptives compared to and combined with physical activity.
Contraception 1997 Feb;55(2):87-90.
Abstract: A cross-sectional study was designed to examine the influence of exercise compared to and in combination with low-dosed oral contraceptives (OCs) on bone mineral density (BMD). One hundred twenty-eight women (20 to 35 years of age) were assigned to four groups with respect to the years of exercise and OC intake. Influence factors were determined by a detailed questionnaire and interview. BMD for L2-4 and the femoral neck was assessed by DXA. The highest BMD values were found in the group of women characterized by long-term exercise (9.45 +/- 4.32 yr) and short use of OC (1.6 +/- 1.69 yr). No beneficial effect of exercise on BMD was found in the group with a long exercise period (10.4 +/- 4.14 yr) and long-term intake of OC (8.2 +/- 4.14 yr). Differences in mean BMD values between the two groups were significant in all regions assessed (p < 0.05). No differences in mean BMD were found in the groups with short-term exercise but long or brief histories of OC. The question arises as to whether active women taking low-dosed OC at an earlier age will develop an adequate BMD.
Heaney RP, Recker RR, Weaver CM. Absorbability of calcium sources: the limited role of solubility.
Calcific Tissue Int 1990;46:300-304.
Hergenroeder AC, Smith EO, Shypailo R, Jones LA, Klish WJ, Ellis K. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone, or placebo over 12 months.
Am J Obstet Gynecol 1997 May;176(5):1017-1025.
Abstract: OBJECTIVES: The objectives of this study were to assess (1) whether treatment with oral contraceptives, in comparison with medroxyprogesterone and placebo, improved bone mineral in women with hypothalamic amenorrhea and (2) whether treatment with medroxyprogesterone, in comparison with placebo, improved bone mineral in women with hypothalamic oligomenorrhea. STUDY DESIGN: The study was a randomized, controlled clinical trial. Twenty-four white women, aged 14 to 28 years, with hypothalamic amenorrhea or oligomenorrhea were prospectively enrolled for a 12-month intervention period. Amenorrheic subjects were randomized to receive oral contraceptives, medroxyprogesterone, or placebo. Oligomenorrheic subjects were randomized to receive medroxyprogesterone or placebo. Bone mineral was measured by dual-energy x-ray absorptiometry at baseline and at 6 and 12 months. RESULTS: In amenorrheic subjects spine and total body bone mineral measurements at 12 months were greater in the oral contraceptive group than in the medroxyprogesterone and placebo groups when baseline bone mineral measurements, body weight, and age were controlled for (p < or = 0.05). There were no differences in hip bone mineral calcium and bone mineral density measurements at 12 months among the three groups. In oligomenorrheic subjects there was no detectable improvement in bone mineral associated with medroxyprogesterone use. CONCLUSIONS: This study supports the hypothesis that oral contraceptive use in women with hypothalamic amenorrhea will improve lumbar spine and total body bone mineral.
Holt GA. Food and Drug Interactions. Chicago: Precept Press, 1998.
Hudson JQ, Small RE, Buckley L. J Am Pharm Assoc (Wash) 1998 Nov-Dec;38(6):710-716. Perceptions of pharmacists about adverse effects of corticosteroid therapy: focus on osteoporosis.
Abstract: OBJECTIVE: To assess the perceptions of pharmacists regarding the adverse effects of corticosteroids, in particular corticosteroid-induced osteoporosis. DESIGN: Mailed survey of a random sample of pharmacists. SETTING: Richmond, Virginia. PARTICIPANTS: 350 community and hospital pharmacists. INTERVENTIONS: Not applicable. MAIN OUTCOME MEASURES: Respondents' knowledge of adverse effects of corticosteroid therapy in men, premenopausal women, and postmenopausal women; the content of respondents' usual patient counseling for low- and high-dose therapy; and respondents' opinions of regimens for prevention of osteoporosis. RESULTS: Pharmacists associated gastritis, weight gain, and mood changes with corticosteroid use in a hypothetical 45-year-old man or 45-year-old premenopausal woman. For a hypothetical 65-year-old postmenopausal woman, pharmacists more frequently counseled about weight gain, osteoporosis, and gastritis. Patient counseling focused on these adverse effects for both low-dose (5 to 10 mg/day) and high-dose (> or = 30 mg/day) prednisone use. Osteoporosis was considered more likely in patients receiving high-dose corticosteroids on a long-term basis. CONCLUSION: Pharmacists responding to this survey frequently overlooked the association between low- and high-dose corticosteroid use and decreased bone density. Educational efforts are needed so that pharmacists can fulfill their potential for educating patients, monitoring corticosteroid therapy, and detecting drug-induced complications.
Humes HD, Sastrasinh M, Weinberg JM. Calcium is a competitive inhibitor of gentamicin-renal membrane binding interactions and dietary calcium supplementation protects against gentamicin nephrotoxicity.
J Clin Invest 1984 Jan;73(1):134-147.
Abstract: The divalent cations, Ca++ and Mg++, are known to competitively inhibit a large number of aminoglycoside-membrane interactions, so that Ca++ prev ents both the neurotoxic and ototoxic effects of these antibiotics acutely in vitro. Since gentamicin-induced plasma and subcellular membrane damage appear to be critical pathogenetic events in gentamicin nephrotoxicity, Ca++ may play a similar protective role in gentamicin-induced acute renal failure. To test this possibility in vivo, rats (group 2) were given a 4% calcium (in the form of CaCO3) supplemented diet to increase delivery of Ca++ to the kidney and administered single daily subcutaneous injections of gentamicin, 100 mg/kg, for 10 d. Compared with a simultaneously studied group (group 1) of rats receiving identical gentamicin dosages and normal diets, Ca++ supplementation ameliorated gentamicin-induced acute renal failure. After 10 doses of gentamicin, blood-urea nitrogen values in group 1 averaged 213 +/- 15 (SE) and 25 +/- 3 (P less than 0.001) in group 2. The progressive decline in renal excretory function, as measured by BUN, in group 1 animals was accompanied by simultaneous declines in renal cortical mitochondrial function and elevations in renal cortex and mitochondrial Ca++ content, quantitative indices of the degree of renal tubular cell injury. Oral Ca++ loading markedly attenuated these gentamicin-induced derangements. After eight and 10 doses of gentamicin, mitochondria isolated from the renal cortex of group 2 rats had significantly higher rates of respiration supported by pyruvate-malate, succinate and N,N,N',N'-tetramethyl-p-phenyldiamine-ascorbate, higher rates of dinitrophenol-uncoupled respiration and greater acceptor control ratios than those measured in mitochondria isolated from the renal cortex of group 1 animals. Similarly, after 8 and 10 doses, renal cortex and renal cortical mitochondrial Ca++ content of group 2 was significantly lower than values observed in group 1. Thus, dietary calcium supplementation significantly protected against gentamicin-induced renal tubular cell injury and, consequently, gentamicin-induced acute renal failure. The mechanism for this protective effect of Ca++ may relate to the manner in which this polycationic antibiotic interacts with anionic sites, primarily the acidic phospholipids of renal membranes. In this regard, Ca++ was found to be a competitive inhibitor both of 125I-gentamicin binding to renal brush border membranes, the initial site of interaction between gentamicin and renal proximal tubule cells, with a composite inhibition constant (Ki) of 12 mM and of 125I-gentamicin binding to phosphatidic acid, an important membrane acidic phosphate.
Iivanainen M, Savolainen H. Side effects of phenobarbital and phenytoin during long-term treatment of epilepsy.
Acta Neurol Scand Suppl 1983;97:49-67.
Abstract: Phenobarbital and phenytoin have good antiepileptic effect, but clinically significant untoward effects occur during their long-term use. Phenobarbital may cause hyperactivity, behavioral problems, sedation, and even dementia; these effects are dose related to some extent. Side effects of phenytoin include sedation, a cerebellar syndrome, phenytoin encephalopathy, psychosis, locomotor dysfunction, hyperkinesia, megaloblastic anemia, decreased serum folate level, decreased bone mineral content, liver disease, IgA deficiency, gingival hyperplasia, and a lupus-like hypersensitivity syndrome. Especially susceptible to the neurotoxic effects of phenytoin are epileptic children with severe brain damage who are on multiple drugs. In those children, balance disturbance may develop and be followed by gradual loss of locomotion. Among 131 mentally retarded epileptic patients, phenytoin intoxication occurred in 73 (56%), of whom 18 experienced persistent loss of locomotion. There is experimental evidence that the toxic action of phenytoin lies at the cellular level, predominantly in the cerebellum. Many experts avoid the long-term use of phenytoin because of its insidious and potentially dangerous side effects.
Jameson SJ, Hargarten SW. Calcium pretreatment to prevent verapamil-induced hypotension in patients with SVT.
Ann Emerg Med 1992 Jan;21(1):68. (Editorial)
Jones G, Sambrook PN. Drug-induced disorders of bone metabolism. Incidence, management and avoidance.
Drug Saf 1994 Jun;10(6):480-489.
Abstract: Calcium homeostasis depends upon the interplay of intestinal calcium absorption, renal excretion and skeletal mobilisation of calcium, mediated through bone formation and resorption, which are closely coupled in the adult skeleton. Serum calcium is extremely important for maintenance of normal cellular functions and is regulated by the major calciotropic hormones, parathyroid hormone (PTH), 1,25-dihydroxy-vitamin D and calcitonin. Certain drugs can interfere with calcium metabolism by effects at different stages in calcium metabolism, and a knowledge of the mechanism of drug action is generally helpful in understanding the various resultant clinical skeletal syndromes. Corticosteroids, for example, have profound effects at multiple stages of calcium metabolism, resulting in decreased bone formation and enhanced bone resorption leading to accelerated osteoporosis. Drugs such as aluminium and anticonvulsants impair mineralisation, leading to osteomalacia. Other drugs, such as fluoride, are employed for their known effects on bone, but in excess dosage can be harmful by producing mineralisation defects. Management of these conditions will be discussed in this review.
Jung H, Peregrina AA, Rodriguez JM, Moreno-Esparza R. The influence of coffee with milk and tea with milk on the bioavailability of tetracycline. Biopharm Drug Dispos 1997 Jul;18(5):459-463.
Abstract: The effect of milk added to coffee or black tea on the bioavailability of tetracycline was evaluated in 12 healthy volunteers according to a crossover design. Results showed that even a small volume of milk containing extremely small amounts of calcium severely impair the absorption of the drug, so that the presence of this metal ion should be carefully controlled in order to avoid decreasing the available tetracycline.
Kes P, Reiner Z. Symptomatic hypomagnesemia associated with gentamicin therapy.
Magnes Trace Elem. 1990;9(1):54-60.
Abstract: Seven patients (3 females, 4 males) developed symptomatic hypomagnesemia, hypocalcemia, and hypokalemia following gentamicin therapy. The excessive and inappropriate urinary excretion of magnesium and potassium in the presence of subnormal serum concentrations was noted. A significant correlation was found between the total cumulative dose of gentamicin and serum Mg concentration (r = 0.76, p less than 0.05), as well as between the renal wasting of Mg and the total cumulative dose of gentamicin administered (r = 0.89, p less than 0.01). The gentamicin-induced Mg depletion is a very rare but important complication which is most likely to occur when the drug is given to older patients in large doses over extended periods of time.
Kindmark A, Rollman O, Mallmin H, Petren-Mallmin M, Ljunghall S, Melhus H
Oral isotretinoin therapy in severe acne induces transient suppression of biochemical markers of bone turnover and calcium homeostasis.
Acta Derm Venereol 1998 Jul;78(4):266-269.
Abstract: Although dietary vitamin A is required for normal growth and development, long-term or high-dose administration of vitamin A derivatives (retinoids) may produce a variety of skeletal side-effects in man. In this study we investigated the early effects of oral isotretinoin therapy on bone turnover and calcium homeostasis in eleven consecutive patients with nodulocystic acne. The effects on bone metabolism were correlated to radiological and bone mineral density measurements following drug therapy for six months. Markers of bone turnover, i.e. serum osteocalcin, the carboxyterminal propeptide of type I collagen, bone specific alkaline phosphatase, the carboxyterminal telopeptide of type I collagen, and urine levels of calcium and hydroxyproline decreased significantly within five days of treatment (p < 0.05). There was also a statistically significant decrease in serum calcium, with a minimum on day five, and a marked increase in serum parathyroid hormone (p < 0.05). With continued treatment, however, the abnormal levels of these markers returned to baseline values within 14 days. No significant roentgenological changes or effects on bone mineral density were found in response to the drug. The observed inhibitory effects of isotretinoin on bone turnover, despite elevated parathyroid hormone levels, indicates that the drug exerts a direct effect on bone tissue.
Kosek JC, Mazze RI, Cousins MJ. Nephrotoxicity of gentamicin. Lab Invest. 1974 Jan;30(1):48-57.
Kuhn M, Schriger DL. Low-dose calcium pretreatment to prevent verapamil-induced hypotension.
Am Heart J 1992 Jul;124(1):231-232.
Kung AWC, Pun KK. Bone mineral density in premenopausal women receiving long-term physiological doses of levothyroxine.
JAMA May 22-29;265(20):2688-2691.
Abstract: Total body and regional bone mineral density (BMD) levels were determined in 26 premenopausal women with Hashimoto's thyroiditis receiving long-term physiological doses of levothyroxine sodium replacement therapy. The BMD levels of each patient were compared with the mean of the BMD levels of age-matched normal controls. The mean levothyroxine sodium dose was 111 +/- 6 micrograms/d, and the mean duration of treatment was 7.5 +/- 5.3 years (range, 1 to 24 years). Dietary calcium intake was similar in both groups, as were serum thyroxine, triiodothyronine, free thyroxine index, and thyrotropin levels. Women receiving the levothyroxine treatment had normal total body BMD levels but had significantly lower BMD levels at the femoral neck (-5.7%), femoral trochanter (-7.0%), Ward's triangle (-10.6%), both arms (right, -7.8%; left, -8.9%), and pelvis (-4.9%). In contrast, lumbar spine BMD levels were similar in the two groups. There was no correlation between the total body or different regional BMD levels and the duration or dosage of levothyroxine treatment or thyroid function test results. However, the z score of the femoral neck of these patients showed a significant negative correlation with their serum free thyroxine index levels. We conclude that patients receiving physiological doses of levothyroxine may have decreased bone density. Thyroid functions in patients receiving long-term levothyroxine treatment should be closely monitored and bone densitometry should be performed in patients at risk for osteoporosis.
Kung AW, Lorentz T, Tam SC. Thyroxine suppressive therapy decreases bone mineral density in post-menopausal women.
Clin Endocrinol (Oxf) 1993 Nov;39(5):535-540.
Abstract: OBJECTIVE: Hyperthyroidism is associated with increased bone turnover and decreased bone mass. This study aimed to evaluate the bone mineral density (BMD) of post-menopausal women on long-term thyroxine suppressive therapy. DESIGN: An age and sex-matched cross-sectional study. PATIENTS: Thirty-four post-menopausal women with carcinoma of thyroid, post total thyroidectomy and 131I ablation, on L-T4 for 12.2 +/- 6.6 years (mean +/- SD). Controls were 34 age-matched healthy Southern Chinese women. MEASUREMENTS: Total body and regional BMDs were determined by dual-energy X-ray absorptiometry. Bone turnover was assessed by biochemical markers. RESULTS: In the thyroxine treated group, total body mineral content was significantly lower than the controls (1652 +/- 356 vs 1994 +/- 270 g mean +/- SD, P < 0.005). They also had lower BMDs in the lumbar spine, femoral neck, trochanter and Ward's triangle (0.75 +/- 0.15 vs 0.92 +/- 0.16 g/cm2, P < 0.005; 0.62 +/- 0.12 vs 0.70 +/- 0.12 g/cm2, P < 0.01; 0.55 +/- 0.14 vs 0.63 +/- 0.15 g/cm2, P < 0.001; 0.55 +/- 0.14 vs 0.63 +/- 0.14 g/cm2, P < 0.005 respectively.) The thyroxine treated group also had higher serum alkaline phosphatase and osteocalcin levels as well as urinary hydroxyproline excretion, suggesting that they had high turnover bone loss. However, the Z-scores of the various regional BMDs were correlated only with the serum osteocalcin level and showed no correlation with the serum thyroxine level or with the dosage or duration of thyroxine treatment. CONCLUSION: Long-term thyroxine suppressive therapy was associated with bone loss and preventive therapy may be indicated in these post-menopausal women at risk of osteoporosis.
Kupfer S, Kosovsky JD. Effects of cardiac Glycosides on renal tubular transport of calcium, magnesium inorganic phosphate and glucose in the dog.
J Clin Invest 1965 44:1132-1143.
Leedman PJ, Stein AR, Chin WW, Rogers JT. Thyroid hormone modulates the interaction between iron regulatory proteins and the ferritin mRNA iron-responsive element.
J Biol Chem 1996 May 17;271(20):12017-12023.
Abstract: The cytoplasmic iron regulatory protein (IRP) modulates iron homeostasis by binding to iron-responsive elements (IREs) in the transferrin receptor and ferritin mRNAs to coordinately regulate transferrin receptor mRNA stability and ferritin mRNA translational efficiency, respectively. These studies demonstrate that thyroid hormone (T3) can modulate the binding activity of the IRP to an IRE in vitro and in vivo. T3 augmented an iron-induced reduction in IRP binding activity to a ferritin IRE in RNA electrophoretic mobility shift assays using cytoplasmic extracts from human liver hepatoma (HepG2) cells. Hepatic IRP binding to the ferritin IRE also diminished after in vivo administration of T3 with iron to rats. In transient transfection studies using HepG2 cells and a human ferritin IRE-chloramphenicol acetyltransferase (H-IRE-CAT) construct, T3 augmented an iron-induced increase in CAT activity by approximately 45%. RNase protection analysis showed that this increase in CAT activity was not due to a change in the steady state level of CAT mRNA. Nuclear T3-receptors may be necessary for this T3-induced response, because the effect could not be reproduced by the addition of T3 directly to cytoplasmic extracts and was absent in CV-1 cells which lack T3-receptors. We conclude that T3 can functionally regulate the IRE binding activity of the IRP. These observations provide evidence of a novel mechanism for T3 to up-regulate hepatic ferritin expression, which may in part contribute to the elevated serum ferritin levels seen in hyperthyroidism.
Lems WF, Van Veen GJ, Gerrits MI, Jacobs JW, Houben HH, Van Rijn HJ, Bijlsma JW. Effect of low-dose prednisone (with calcium and calcitriol supplementation) on calcium and bone metabolism in healthy volunteers.
Br J Rheumatol 1998 Jan;37(1):27-33.
Abstract: The administration of moderate to high doses of corticosteroids is associated with bone loss. This probably results from the uncoupling of bone formation (decreased) and bone resorption (unchanged or increased). We examined the effect of low-dose (10 mg/day) prednisone (LDP) and the possible mitigating effects of calcium and 1.25 (OH)2 vitamin D (calcitriol) on calcium and bone metabolism in eight healthy, young male volunteers. The study consisted of four observation periods: in the first period, LDP was prescribed during 1 week; in the second, third and fourth periods, calcium (500 mg/day), calcitriol (0.5 micrograms b.i.d.) and calcium in combination with calcitriol, respectively, were added to LDP. Bone formation was measured by means of serum osteocalcin, carboxy-terminal propeptide of type 1 procollagen (P1CP) and alkaline phosphatase, bone resorption by means of urinary excretion of calcium, hydroxyproline, (free and total) pyridinoline, (free and total) deoxypyridinoline and serum carboxy-terminal cross-linked telopeptide of type 1 collagen (1CTP). Dietary calcium and sodium intake were maintained at a stable level during the entire study period. Treatment with LDP led to a decrease in osteocalcin, P1CP and alkaline phosphatase (all P < 0.01). Urinary excretion of pyridinolines, hydroxyproline and serum 1CTP did not increase, but remained unchanged or slightly reduced (P < 0.05), depending on the time of measurement and the marker of bone resorption. Parathyroid hormone (PTH) (insignificantly) increased during LDP (+19%) and LDP plus calcium (+14%), but decreased during supplementation with calcitriol (-16%) and calcium/calcitriol (-44%; P < 0.01). Urinary excretion of calcium increased during treatment with LDP and calcitriol (P < 0.05) and calcium/calcitriol (P < 0.05). It is concluded that LDP has a negative effect on bone metabolism, since bone formation decreased while bone resorption remained unchanged or decreased slightly. The increase in PTH during LDP could be prevented by calcitriol combined with calcium supplementation.
Leonard JP, Desager JP, Beckers C, Harvengt C. In vitro binding of various biological substances by two hypocholesterolaemic resins. Cholestyramine and colestipol.
Arzneimittelforschung 1979;29(7):979-981.
Abstract: The ability of cholestyramine and colestipol, two hypocholesterolaemic resins, to bind in vitro several compounds such as vitamin B12, vitamin B12-intrinsic factor complex, folic acid, iron citrate and calcium chloride was investigated. Both resins bound to a high extent vitamin B12-intrinsic factor complex, folic acid and iron citrate; in addition, cholestyramine also caused appreciable binding of calcium. Throughout a large range of pH, there was no change in the binding capacity; however, at pH 2, cholestyramine exhibited a marked drop in the binding of tested substances (with exception of folic acid). By increasing the molarity of the solutions, the binding to the resins of vitamin B12-intrinsic factor complex and of calcium chloride was completely inhibited. In human gastric and duodenal juices, the uptake by the resins of the studied compounds depends on the molarity of the physiological medium tested and partly confirms the results obtained with aqueous solutions. These data obtained in vitro emphasize the necessity of regular monitoring these biochemical parameters during chronic treatment of hypercholesterolaemia conducted with these two resins.
Lim D, McKay M. Food-drug interactions. Drug Information Bull UCLA Department of Pharmaceutical Services. 1995;15(2). (Review).
Lindberg JS, Copley JB, Koenig KG, Cushner HM. Effect of citrate on serum aluminum concentrations in hemodialysis patients: a prospective study.
South Med J. 1993 Dec;86(12):1385-1388.
Abstract: Twenty hemodialysis patients were prospectively evaluated to determine if concomitant citrate and aluminum administration enhances the absorption of aluminum, thereby increasing the possibility of toxicity. The four-phase study consisted of phase I, a washout phase; phase II, an aluminum treatment phase; phase III, a treatment phase combining aluminum and soluble calcium citrate; and phase IV, a treatment phase with the patient's original prestudy phosphate binder. Results disclosed a progressive rise in serum aluminum levels (microgram/L) from 47 +/- 8 (phase I) to 62 +/- 12 (phase II) to 74 +/- 13 (phase III) and a drop to 58 +/- 12 (phase IV). The difference in levels between phases I and III was significant. Additionally, and despite the fact that serum calcium concentrations did not change, serum phosphate and immunoreactive parathyroid hormone concentrations were significantly lower when aluminum and citrate were used together. This suggests that citrate enhances the absorption of aluminum and therefore increases the possibility of toxicity in the patient with end-stage renal disease.
Liu T, Soong SJ, Wilson NP, Craig CB, Cole P, Macaluso M, Butterworth CE Jr. A case control study of nutritional factors and cervical dysplasia.
Cancer Epidemiol Biomarkers Prev 1993 Nov-Dec;2(6):525-530.
Abstract: The association of nutritional factors with cervical dysplasia was examined through a case-control study. Analysis was conducted in 257 cases and 133 controls confirmed both by cytological examination and colposcopic findings. A 24-h dietary recall questionnaire was used to assess nutritional intake. Various risk factors (including age at first intercourse, number of sexual partners, parity, cigarette smoking, oral contraceptive use, human papillomavirus type 16 infection, and age and race) were adjusted for their potential confounding effects. While analyses were also performed to adjust for total calories, results were not changed significantly. Among the nutrients examined, vitamin A intake showed a significantly increased risk at the lowest quartile compared to the highest quartile, with an odds ratio of 2.2 (95% confidence interval, 1.2-4.2). A significant trend of increasing risk was also observed with lower intake of vitamin A (P = 0.05). Riboflavin showed increased risk at the two lower quartiles of intake with a trend test P value of 0.04. Increased risk was also found for lower intakes of vitamin C compared to the highest intake level. For folate, increased risk was found in the second highest quartile compared with the highest quartile with an odds ratio of 2.0 (95% confidence interval, 1.0-3.8). The calcium:phosphorus ratio showed an increased risk at the lowest level (odds ratio, 2.0; 95% confidence interval, 1.0-4.3). Insufficient intake of vitamin A, riboflavin, ascorbate, and folate is associated with an increased risk of cervical dysplasia.
Lomaestro BM, Bailie GR. Effect of multiple staggered doses of calcium on the bioavailability of ciprofloxacin.
Ann Pharmacother 1993 Nov;27(11):1325-1328.
Abstract: OBJECTIVE: To determine the effect on the relative bioavailability (Fr) of a staggered single dose of ciprofloxacin given two hours after a morning dose of calcium carbonate given three times daily over the three previous days. DESIGN: Thirteen male volunteers participated in this randomized, nonblinded, crossover investigation; 12 subjects were included in the final analysis. SETTING: Data collection and ciprofloxacin administration occurred at Albany Medical Center, a tertiary-care teaching institution. Calcium carbonate administration was on an outpatient basis. RESULTS: For 12 volunteers, the mean +/- SD Fr of ciprofloxacin staggered with calcium was 0.87 +/- 0.23 (noncompartmental model) and 0.98 +/- 0.27 (compartmental model). Other statistically significant findings were a decrease in the time to maximum concentration of ciprofloxacin staggered with calcium in serum compared with ciprofloxacin alone (from 1.76 +/- 0.54 to 1.23 +/- 0.52 h in the noncompartmental model; p < 0.05), and a decrease in the same parameter (from 1.92 +/- 0.96 to 0.77 +/- 0.53 in the compartmental model; p < 0.005). Maximum concentration of ciprofloxacin staggered with calcium was decreased in the noncompartmental model compared with ciprofloxacin alone (from 2.11 +/- 0.72 to 1.60 +/- 0.33, respectively; p < 0.05). The elimination half-life and area under the concentration-time curve of ciprofloxacin were not significantly altered. CONCLUSIONS: Repeated doses of calcium carbonate, administered two hours before ciprofloxacin, did not significantly alter the Fr of this fluoroquinolone.
Lund B, Andersen RB, Friis T, Hjorth L, Jorgensen FS, Norman AW, Sorensen OH. Effect of 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on intestine and bone in glucocorticoid-treated patients.
Clin Endocrinol (Oxf) 1977 Dec;7 Suppl:177s-181s.
Abstract: The effect of 1alpha-hydroxyvitamin D3 (1alpha-OHD3) and 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3) on the intestinal calcium absorption was studied in twenty patients with rheumatoid arthritis treated with prednisone at daily doses of 5--15 mg for 1/2--20 years. The fractional calcium absorption, measured before and after the treatment with the vitamin D compounds, increased in nineteen of the twenty patients. This was, however, accompanied by marked rises in the urinary calcium excretion. There was no correlation between the fractional calcium absorption and the duration of the prednisone treatment or the doses given.
Lopez Alvarez MB, Hawkins F, Rigopoulou D, Martinez G, Jodar E, Estenoz J, Ortuno B, Arnaiz F. [The risk factors and bone mineral density in women on long-term levothyroxine treatment].
Med Clin (Barc) 1999 Jan 30;112(3):85-89. [Article in Spanish]
Abstract: BACKGROUND: It is controversial if the long-term treatment with thyroid hormone given at substitutive or suppressive doses has a negative effect on bone metabolism. In previous reports the lack of ultrasensitive TSH assays and densitometers with adequate precision, and the heterogeneity of the patients analyzed could explain these discordant results. PATIENTS AND METHODS: We have assessed bone mineral density (BMD) in 43 premenopausal and 53 postmenopausal women, who underwent near total thyroidectomy and I-131 ablation due to differentiated thyroid cancer, that have been followed up (mean duration, 75.5 [43] months) with suppressive thyroid hormone treatment (mean dose, 170 [42] micrograms) in our hospital. Patients with history of hyperthyroidism were excluded. Lumbar BMD (L2-L4) and BMD in three different sites of hip were measured (dual X-ray densitometry) to determine the contribution of several clinical and risk factors associated with thyroid hormone therapy given to BMD. RESULTS: We have not found significant decrease in BMD at spine or hip when patients were compared with healthy, age and sex matched. Age (inverse correlation) and weight (direct correlation) were the variables mostly influencing BMD). Histologic type of thyroid neoplasia, doses of thyroid hormones, thyroid hormone levels and duration of follow-up, were not associated with changes in BMD. A decrease in calcium intake in postmenopausal and less physical activity in premenopausal women were related with a decreased lumbar BMD. CONCLUSIONS: During long-term treatment of female patients with thyroid hormones, other risk factors should be studied in order to prevent possible loss of bone mass.
Macallan DC, Maxwell JD, Eastwood JB. Osteomalacia should be sought and treated before withdrawal of anticonvulsant therapy in UK Asians.
Postgrad Med J 1992 Feb;68(796):134-136.
Abstract: Individuals from the Asian sub-continent in the United Kingdom are at particular risk of developing osteomalacia. We report a Gujarati woman who developed osteomalacia whilst taking anticonvulsant drugs; withdrawal of anticonvulsant therapy was followed by a seizure complicated by femoral neck fracture. In patients with other risk factors for osteomalacia, as is the case for Asians living in Britain, anticonvulsant drugs should not be reduced or withdrawn until osteomalacia, which puts the skeleton at increased risk of fracture, and its associated hypocalcaemia, which reduces seizure threshold, have been sought and adequately treated.
Majerus PW, Broze GJ Jr, Miletich JP, Tollefsen DM. Anticoagulant, thrombolytic, and antiplatelet drugs. In:
Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 9th ed. New York: McGraw-Hill 1996, 1346.
Mazze RI, Cousins MJ. Combined nephrotoxicity of gentamicin and methoxyflurane anaesthesia in man. A case report.
Br J Anaesth. 1973 Apr;45(4):394-398.
Mazze RI, Cousins MJ. Combined nephrotoxicity of gentamicin and methoxyflurane anaesthesia in man. A case report.
Br J Anaesth. 1973 Apr;45(4):394-398.
McLean, R. Magnesium and its therapeutic uses: A review. Am J Med 1994 Jan;96(1):63-76. (Review)
Montie T, Patamasucon P. Aminoglycosides: the complex problem of antibiotic mechanisms and clinical applications.
Eur J Clin Microbiol Infect Dis 1995;14:85-87. (Editorial)
Nelson-Piercy C. Heparin-induced osteoporosis in pregnancy. Lupus 1997;6(6):500-504. (Review)
Nesbitt LT Jr. Minimizing complications from systemic glucocorticosteroid use.
Dermatol Clin 1995 Oct;13(4):925-939. (Review)
Abstract: For proper use of systemic GCS, a basic knowledge of the normal HPA axis, as well as knowledge of the pharmacology, clinical usage guidelines, and adverse reactions of these agents is imperative. Both short-term (acute) and long-term side effects should be well known by the physician. The pros and cons of oral and parenteral therapy for various disorders and in various situations should be recognized. For long-term therapy, an intermediate-acting agent such as prednisone in single, early morning doses is most commonly used to minimize suppression of the HPA axis. Alternate-morning doses produce even less suppression if the disease process will respond. A through patient history, including general medical history and medications the patient is taking, is important to anticipate any potential problems. Weight and blood pressure should be checked initially and every 1 to 3 months thereafter. Blood glucose, electrolytes, and lipid studies, including triglycerides, should be done approximately every 6 months. An ophthalmology examination should be performed every year, and stool examination for occult blood and chest radiography can be obtained as indicated. Bone density studies might be necessary in patients who are at high risk for osteoporosis. Specific acute situations may dictate other studies. The patient on long-term GCS should be kept as active as possible, as mild-to-moderate exercise helps prevent certain side effects, such as osteoporosis. The dose of oral GCS is best given with food to prevent gastrointestinal irritation, and agents to decrease gastric acidity might be needed in certain situations. Exposure to infections should be prevented, where possible, and treatment initiated at the first sign of systemic or cutaneous infection. Pain should be evaluated early, especially abdominal pain or bone pain; MRI is indicated if aseptic necrosis of bone is suspected. Both trauma and severe sun exposure should be avoided. Consultation with other specialists is strongly recommended when the situation dictates. Diet is one of the most important strategies to minimize side effects from long-term GCS therapy. Vegetable protein should be increased in the diet, and fats and carbohydrates limited. Adequate calcium is imperative, and calcium supplementation is recommended for high-risk osteoporosis patients. Small amounts of vitamin D may be necessary to increase absorption of calcium. Restriction of sodium is also important, as is maintainance of dietary potassium. Supplemental potassium may be necessary in some patients, and a thiazide diuretic might be useful in patients with hypertension, edema, or osteoporosis. Vitamin C can be given to promote wound healing. A good doctor-patient relationship is important in managing the patient on long-term GCS. The patient must return for regular visits and be encouraged to promptly report any adverse reactions to the physician. If these criteria are maintained and the strategies noted previously are followed, problems from long-term therapy with GCS will be minimized.
Nolan CR, DeGoes JJ, Alfrey AC. Aluminum and lead absorption from dietary sources in women ingesting calcium citrate.
South Med J. 1994 Sep;87(9):894-898.
Abstract: Animal models suggest that citrate-containing compounds augment absorption of aluminum from food and tap water, causing aluminum accumulation in bone and brain despite normal renal function. Citrate also enhances lead absorption in animals. We questioned whether use of calcium citrate by women as a calcium supplement causes an increase in aluminum or lead absorption from dietary sources. Changes in 24-hour urine aluminum and lead excretion, plasma aluminum level, and whole blood lead level were assessed in 30 healthy women before and during treatment with calcium citrate (800 mg of elemental calcium per day). During calcium citrate therapy, urinary aluminum excretion and plasma aluminum level increased significantly. In contrast, there were no changes in urine or whole blood lead levels. We conclude that treatment with calcium citrate significantly increases absorption of aluminum from dietary sources. Additional studies are needed to determine whether long-term use of calcium citrate leads to aluminum accumulation and toxicity.
Nolan CR, Califano JR, Butzin CA. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption.
Kidney Int. 1990 Nov;38(5):937-941.
Abstract: The risk of aluminum (Al) accumulation in patients with chronic renal failure has led to use of non-Al phosphate binders. Frequently, Al and non-Al phosphate binders are co-administered. Unfortunately, calcium citrate (Ca citr), when given with Al-gel, markedly enhances Al absorption. To determine whether calcium acetate (Ca acetate) also augments Al absorption, 10 normal volunteers were each given orally, three-day courses of the following drug combinations dosed four times daily: 1) aluminum hydroxide gel (Al[OH]3) (5 ml) alone; 2) Al[OH]3 (5 ml) plus Ca acetate (1330 mg); 3) Al[OH]3 (5 ml) plus Ca citr (950 mg). A nine day wash-out occurred between each course. Al levels were measured using flameless atomic absorption spectrophotometry. Daily urine Al excretion was measured during a two-day baseline before each course and during each three-day drug course. Plasma Al was obtained during each baseline and drug course. Mean 24-hour Al excretion (micrograms/g creatinine/day) at baseline versus treatment for each combination was: 1) 5.9 +/- 3.2 versus 42.0 +/- 40.7 (mean +/- SD); 2) 5.7 +/- 3.0 versus 40.3 +/- 28.6: 3) 6.3 +/- 3.4 versus 175.8 +/- 103.3. Al excretion was significantly greater with combination 3 than with either 1 or 2 (P less than 0.05). The difference between 1 and 2 was not significant. Plasma Al (micrograms/liter) at baseline versus treatment for each combination was: 1) 5.3 +/- 4.2 versus 8.1 +/- 2.5 (mean +/- SD); 2) 3.1 +/- 2.2 versus 7.3 +/- 2.9; 3) 3.0 +/- 2.3 versus 12.0 +/- 6.1.
Nuti R, Vattimo A, Turchetti V, Righi G. 25-Hydroxycholecalciferol as an antagonist of adverse corticosteroid effects on phosphate and calcium metabolism in man.
J Endocrinol Invest 1984 Oct;7(5):445-448.
Abstract: The present study was performed in 30 patients who needed steroid therapy: courses of triamcinolone or DTM 8-15 given orally lasted 30 days. In 15 of these patients glucoactive corticosteroids were administered in combination with 5 micrograms/day of 25OH-vitamin D3 (25OHD3). 47Calcium oral test and 99mTc-MDP kinetics, as an index of bone turnover, were performed at the beginning of the therapy and after 30 days. At the end of treatment a significant improvement of intestinal radiocalcium transport together with a decrease in bone turnover in the group of patients treated with 25OHD3 was observed. As it concerns plasma calcium level, inorganic phosphate, the urinary excretion of calcium, phosphate and hydroxyproline no significant difference between the two groups examined were noticed. These results indicate that the adverse effects of glucoactive corticosteroids on intestinal calcium transport and bone turnover may be counteracted by the combined administration of physiological doses of 25OHD3.
Nuzzo V, Lupoli G, Esposito Del Puente A, Rampone E, Carpinelli A, Del Puente AE, Oriente P. Bone mineral density in premenopausal women receiving levothyroxine suppressive therapy.
Gynecol Endocrinol 1998 Oct;12(5):333-337.
Abstract: Osteoporosis is a well-known complication of thyrotoxicosis. Prolonged subclinical hyperthyroidism due to L-thyroxine treatment has been associated with reduced bone mass and thus with the potential risk of premature development of osteoporosis. The aim of this study was to assess the effect of a chronic L-thyroxine suppressive treatment on bone mineral density (BMD) in a group of premenopausal women. Forty consecutive patients (mean age +/- SE = 40.95 +/- 1.56 years) affected by non-toxic goiter underwent bone mineral densitometry (dual energy X-ray absorptiometry; DEXA) of the lumbar spine (L1-L4) and right femoral neck. At the time of the study the patients had been under thyroid stimulating hormone (TSH) suppressive therapy for 74.95 +/- 10.34 months (range 17-168 months). Baseline levels of free thyroxine (fT4), free triiodothyronine (fT3), TSH, calcium and phosphorus were measured and correlated with BMD. The age of starting, duration of treatment, main daily dose, cumulative dose of treatment and body mass index (BMI) were also correlated with BMD. Statistical analysis was performed by multiple linear regression. BMD among female patients was not significantly different from that of the general population matched for age and sex. With the use of the regression model, no significant correlation was found between BMD and the variables considered. In conclusion, our data suggest that L-thyroxine suppressive therapy, if carefully carried out and monitored, has no significant effect on bone mass.
O'Regan S, Chesney RW, Hamstra A, Eisman JA, O'Gorman AM, Deluca HF. Reduced serum 1,25-(OH)2 vitamin D3 levels in prednisone-treated adolescents with systemic lupus erythematosus. Acta Paediatr Scand 1979 Jan;68(1):109-111.
Abstract: The serum levels of 1,25-(OH)2 vitamin D3 were assayed in samples from 12 adolescent patients with SLE. Subnormal levels were observed in 7 of these 12 patients. Low levels of the metabolically active polar metabolite of vitamin D3 may contribute to the development of osteopenia observed in this disease. The cumulative effects of the osteoporotic and anti vitamin D effects of long term steroid therapy in children with SLE may require the cautious administration of supplemental vitamin D.
Parsons PP, Garland HO, Harpur ES, Old S. Acute gentamicin-induced hypercalciuria and hypermagnesiuria in the rat: dose-response relationship and role of renal tubular injury.
Br J Pharmacol 1997 Oct;122(3):570-576.
Abstract: 1. Standard renal clearance techniques were used to assess the dose-response relationship between acute gentamicin infusion and the magnitude of hypercalciuria and hypermagnesiuria in the anaesthetized Sprague-Dawley rat. Also investigated were whether these effects occurred independently of renal tubular cell injury. 2. Acute gentamicin infusion was associated with a significant hypercalciuria and hypermagnesiuria evident within 30 min of drug infusion. The magnitude of these responses was related to the dose of drug infused (0.14-1.12 mg kg(-1) min[-1]). Increased urinary electrolyte losses resulted from a decreased tubular reabsorption of calcium and magnesium. 3. A rapid dose-related increase in urinary N-acetyl-beta-D-glucosaminidase (NAG) excretion was also observed in response to gentamicin infusion. However, there was no evidence of renal tubular cell injury and no myeloid bodies were observed within the lysosomes of the proximal tubular cells. Gentamicin may thus interfere with the mechanisms for cellular uptake and intracellular processing of NAG causing increased NAG release into the tubular lumen. 4. The absence of changes in renal cellular morphology indicates that the excessive renal losses of calcium and magnesium were an effect of gentamicin per se and not the result of underlying renal tubular injury. The renal effects described in this paper were apparent after administration of relatively low total drug doses, and with plasma concentrations calculated to be within the clinical range. These findings suggest that disturbances of plasma electrolyte homeostasis could occur in the absence of overt renal injury in patients receiving aminoglycoside antibiotics.
Paul TL, Kerrigan J, Kelly AM, Braverman LE, Baran DT. Long-term L-thyroxine therapy is associated with decreased hip bone density in premenopausal women.
JAMA 1988 Jun 3;259(21):3137-3141.
Abstract: The effect of long-term L-thyroxine (L-T4) therapy on axial skeleton bone density was studied in 31 premenopausal women; the bone densities of these women were compared with the bone densities of 31 age- and weight-matched women without thyroid or bone abnormalities. The women receiving L-T4 therapy had been receiving the medication for a minimum of five years. There was no difference in calcium intake or excretion between the L-T4-treated women and the controls. Women receiving L-T4 had increased serum thyroxine concentrations (134 +/- 5 vs 95 +/- 3 nmol/L [10.4 +/- 0.4 vs 7.4 +/- 0.2 micrograms/dL]), an increased free thyroxine index (9.4 +/- 0.4 vs 6.8 +/- 0.2), and decreased serum thyroid-stimulating hormone concentrations (0.9 +/- 0.2 mU/L vs 2.1 +/- 0.3 mU/L [0.9 +/- 0.2 vs 2.1 +/- 0.3 microU/mL]). Serum triiodothyronine concentrations were normal and were similar in both groups. Women treated with L-T4 had a 12.8% lower bone density at the femoral neck and a 10.1% lower bone density at the femoral trochanter compared with matched controls. In contrast, lumbar spine bone density was similar in the two groups. The data suggest that long-term L-T4 therapy, which is often given at supraphysiologic dosages, may predispose patients to decreased bone density in the hip and may increase the risk of age-related bone loss. It is advisable, therefore, to employ a dosage of L-T4 that is carefully monitored to avoid the long-term use of dosages that are excessive for the thyroid condition being treated.
Pluskiewicz W, Nowakowska J. Bone status after long-term anticonvulsant therapy in epileptic patients: evaluation using quantitative ultrasound of calcaneus and phalanges.
Ultrasound Med Biol 1997;23(4):553-558.
Abstract: The bone status of 25 epileptic female patients on long-term (mean 19 y) anticonvulsant therapy was investigated using quantitative ultrasound of the calcaneus (Lunar Achilles) and phalanges (Igea DBM Sonic 1200). Comparisons were made with a control group of 43 normal healthy women. Radiogrammetric measurements of the second metacarpal bone were also made in the epileptic patients. While all of the ultrasonic parameters were reduced in the epileptic group, differences only achieved statistical significance for speed of sound (SOS) at the phalanges. Phalangeal SOS correlated significantly with cortical thickness of the second metacarpal bone (r = 0.44, p < 0.05). The data suggest that long-term anticonvulsant therapy is associated with significant cortical bone loss. Quantitative ultrasound may have a role in monitoring bone loss in epileptic patients and in guiding suitable preventive therapy.
Pronsky Z. Powers and Moore's Food-Medications Interactions. Ninth Edition. Food-Medication Interactions. Pottstown, PA, 1991.
Quarum ML, Houghton DC, Gilbert DN, McCarron DA, Bennett WM. Increasing dietary calcium moderates experimental gentamicin nephrotoxicity. J Lab Clin Med 1984 Jan;103(1):104-114.
Abstract: Because calcium has been reported to modify gentamicin binding to its proximal tubular brush border membrane receptor, we studied the effects of dietary calcium loading and subsequent hypercalciuria on experimental gentamicin nephrotoxicity. Male Fischer 344 rats were fed one of two diets that were identical except for calcium carbonate content: normal (0.5%) and high (4%). The high-calcium diet made rats hypercalciuric but there were no differences between the two groups in inulin clearance, sodium or osmolar excretion, or serum calcium prior to gentamicin administration. Animals on both diets were treated with gentamicin, 20 mg/kg b.i.d., for periods of 3 to 21 days. Both groups developed acute renal failure, but animals on the high-calcium diet had less severe acute toxic injury, as evidenced by studies of inulin clearance, renal histology, and in vitro cortical uptake of NMN and PAH. Furthermore, calcium-loaded animals tended to have lower peak renal cortical gentamicin levels during the period of acute toxicity. The mechanism by which increased dietary calcium protects against gentamicin nephrotoxicity remains speculative. Calcium and gentamicin may compete for the same brush border receptor or alternatively parathyroid suppression may result in diminution in tubular cell membrane drug binding sites. The possibility that high-calcium diets exert a nonspecific salutory effect on proximal tubular cell integrity has not been excluded.
Reid IR, Ibbertson HK. Calcium supplements in the prevention of steroid-induced osteoporosis.
Am J Clin Nutr. 1986 Aug;44(2):287-290.
Abstract: The long-term use of glucocorticoid drugs frequently results in the development of osteoporosis. To assess the value of calcium supplementation in preventing this loss of bone, the metabolic effects of administering 1 g of elemental calcium/day have been studied in 13 steroid-treated patients. After 2 mo, the fasting urine hydroxyproline-creatinine ratio decreased from 27.1 +/- 2.5 (SEM) to 21.8 +/- 2.4 (p less than 0.001) and there was an increase in fasting urine-calcium excretion (p less than 0.05). Serum alkaline phosphatase and osteocalcin showed no change. We concluded that calcium supplementation suppresses bone resorption without detectable suppression of indices of bone formation and is, therefore, likely to result in increased bone mass. The safety and low cost of calcium make it a very suitable prophylactic agent in glucocorticoid-treated patients.
Reid IR, Ibbertson HK. Corticosteroids and osteoporosis. Aust N Z J Med. 1987 Dec;17(6):611-612. (Letter)
Rejnmark L, Mosekilde L, Andreasen F. [Diuretics and osteoporosis]. Nord Med 1998 Feb;113(2):53-59. [Article in Danish]
Abstract: Thiazide diuretics lower while loop diuretics promote calcium excretion by the kidney. Several studies have found thiazide use to be associated with higher bone mineral density and some have found that thiazides reduce the risk of hip fracture. The mechanisms by which thiazides favour preservation of the bones are uncertain. Thiazide use results in decreased renal calcium excretion and thiazide users have been shown to have lower levels of S-PTH and S-1,25-dihydroxy-vitamin D. The beneficial bone effects may result from a decrease in PTH-stimulated bone resorption and an associated reduction in the bone turn-over rate. Whether loop diuretics increases the bone turn-over by augmenting the urinary calcium excretion is more controversial as only few studies have been carried out on loop diuretics. However, in these studies the use of loop diuretics have been associated with decreased bone mineral density and increased risk of fractures. Future research should determine the minimal dose of thiazide therapy necessary to produce a sustained hypocalciuric effect and in addition the influence of diuretic dose on bone turn-over. Equally important is the need to evaluate potential unwanted effects of loop diuretics. In the mean time, thiazide diuretics may be used safely while some caution is necessary in the long term use of loop diuretics in patients who are prone to osteoporosis.
Riis B, Christiansen C. Actions of thiazide on vitamin D metabolism: A controlled therapeutic trial in normal women early in the postmenopause.
Metabolism 1985 May;34(5):421-424.
Abstract: The effect of thiazide on vitamin D metabolism in normal postmenopausal women was studied during a twelve-month placebo-controlled clinical study. Nineteen healthy women in their early menopause were randomized for treatment with bendroflumethiazide (5 mg/d) (n = 11) or placebo (n = 8) for twelve months. All participants were given a calcium supplement of 0.5 g/d throughout the study. A significant increase (p less than 0.01) in the serum concentration of 24,25-dihydroxycholecalciferol was observed in the thiazide group. Moreover, this group showed a tendency toward decreased serum 1,25-dihydroxycholecalciferol, whereas the mean serum 25-hydroxycholecalciferol was unchanged. Except for a highly significant decrease in urinary calcium in the thiazide group (P less than 0.01) all other biochemical indices of calcium metabolism were unchanged. The present data indicate that thiazide given to early postmenopausal women has a primary effect on the renal tubules followed by a secondary change in vitamin D metabolism leading to an increase in serum 24,25-dihydroxycholecalciferol.
Ringe JD, Becker K. [Osteoporosis caused by long term heparin therapy]. Med Monatsschr Pharm 1985 Mar;8(3):80-83. [Article in German]
Ringe JD, Keller A. [Risk of osteoporosis in long-term heparin therapy of thromboembolic diseases in pregnancy: attempted prevention with ossein-hydroxyapatite].
Geburtshilfe Frauenheilkd 1992 Jul;52(7):426-429. [Article in German]
Abstract: Generalized idiopathic osteoporosis and transient osteoporosis of the hip are both rare complications of pregnancy. More frequently, long-term heparin administration to treat deep thrombosis in the legs or pelvis may lead to substantial decreases in bone mass and consequently increased risk of osteoporosis. Therapeutic studies with the aim to counteract the osteoporosis inducing effect of heparins, have not been published to date. In the special situation of pregnancy, most medications used for osteoporosis are contraindicated. In our open randomised study, 9 women on heparin-treatment received daily 6.46 g of the bone preparation OHC (ossein-hydroxyapatite-compound) over a period of 6 months and were compared to 11 women without bone protective treatment. In the OHC-group, good compliance was observed with no side effects and reduced back pain. Bone mass did not change significantly, whilst it dropped significantly statistically in the controls.
Robinson C, Weigly E. Basic Nutrition and Diet Therapy. New York: MacMillan, 1984.
Roe DA. Diet and Drug Interactions. New York: Van Nostrand Reinhold, 1989.
Roe DA. Drug-induced Nutritional Deficiencies. 2nd ed. Westport, CT: Avi Publishing, 1985.
Roe DA. Risk factors in drug-induced nutritional deficiencies. In: Roe DA, Campbell T, eds.
Drugs and Nutrients: The Interactive Effects. New York: Marcel Decker, 1984: 505-523.
Rude RK, Gruber HE, Oldham SB. Cortisone-induced osteoporosis: effects on bone adenylate cyclase.
Miner Electrolyte Metab 1993;19(2):71-77.
Rupp WM, McCarthy HB, Rohde TD, Blackshear PJ, Goldenberg FJ, Buchwald H. Risk of osteoporosis in patients treated with long-term intravenous heparin therapy.
Curr Surg 1982 Nov;39(6):419-422.
Quarum ML, Houghton DC, Gilbert DN, McCarron DA, Bennett WM. Increasing dietary calcium moderates experimental gentamicin nephrotoxicity. J Lab Clin Med 1984 Jan;103(1):104-114.
Abstract: Because calcium has been reported to modify gentamicin binding to its proximal tubular brush border membrane receptor, we studied the effects of dietary calcium loading and subsequent hypercalciuria on experimental gentamicin nephrotoxicity. Male Fischer 344 rats were fed one of two diets that were identical except for calcium carbonate content: normal (0.5%) and high (4%). The high-calcium diet made rats hypercalciuric but there were no differences between the two groups in inulin clearance, sodium or osmolar excretion, or serum calcium prior to gentamicin administration. Animals on both diets were treated with gentamicin, 20 mg/kg b.i.d., for periods of 3 to 21 days. Both groups developed acute renal failure, but animals on the high-calcium diet had less severe acute toxic injury, as evidenced by studies of inulin clearance, renal histology, and in vitro cortical uptake of NMN and PAH. Furthermore, calcium-loaded animals tended to have lower peak renal cortical gentamicin levels during the period of acute toxicity. The mechanism by which increased dietary calcium protects against gentamicin nephrotoxicity remains speculative. Calcium and gentamicin may compete for the same brush border receptor or alternatively parathyroid suppression may result in diminution in tubular cell membrane drug binding sites. The possibility that high-calcium diets exert a nonspecific salutory effect on proximal tubular cell integrity has not been excluded.
Sakhaee K, Ruml L, Padalino P, Haynes S, Pak CY. The lack of influence of long-term potassium citrate and calcium citrate treatment in total body aluminum burden in patients with functioning kidneys. J Am Coll Nutr. 1996 Feb;15(1):102-106.
Abstract: BACKGROUND: It has been suggested that citrate salts might enhance aluminum (Al) absorption from a normal diet, posing a threat of Al toxicity even in subjects with normal renal function. We have recently reported that in normal subjects and patients with moderate renal failure, short-term treatment with tricalcium dicitrate (Ca3Cit2) does not significantly change urinary and serum Al levels. However, we have not assessed total body Al stores in patients on long-term citrate treatment. OBJECTIVE: The objective of this study was to ascertain body content of Al non-invasively using the increment in serum and urinary Al following the intravenous administration of deferoxamine (DFO) in patients with kidney stones and osteoporotic women undergoing long-term treatment with potassium citrate (K3Cit) or Ca3Cit2, respectively. METHODS: Ten patients with calcium nephrolithiasis and five with osteoporosis who were maintained on potassium citrate (40 mEq/day or more) or calcium citrate 800 mg calcium/day (40 mEq citrate) for 2 to 8 years, respectively, and 16 normal volunteers without a history of regular aluminum-containing antacid use participated in the study. All participants completed the 8 days of study, during which they were maintained on their regular home diet. Urinary Al excretion was measured during a two-day baseline before (Days 5, 6) and for 1 day (Day 7) immediately following a single intravenous dose of DFO (40 mg/kg). Blood for Al was obtained before DFO administration, and at 2, 5 and 24 hours following the start of the infusion. RESULTS: The median 24-hour urinary Al excretion (microgram/day) at baseline versus post-DFO value was 15.9 vs. 44.4 in the normal subjects and 13.3 vs. 35.7 in the patients. These values were all within normal limits and did not change significantly following DFO infusion (p = 0.003 and p = 0.0001, respectively). The median change of 17.1 micrograms/day in urinary Al in the normal subjects was not significantly different from the 18.7 micrograms/day change measured in the patient group (p = 0.30). Similarly, no change in the mean serum Al was detected at any time following the DFO infusion, either in the patient or control group (patients 4.1 to 4.3 ng/ml, controls 7.4 to 4.6 ng/ml). CONCLUSION: The results suggest that abnormal total body retention of Al does not occur during long-term citrate treatment in patients with functioning kidneys.
Salazar T, Barrera F, Capurro MT, Barra C, Salinas A, Novoa F. [Serum concentrations of phenobarbital and the metabolism of calcium and phosphorus in children].
Rev Chil Pediatr 1984 Nov-Dec;55(6):407-410. [Article in Spanish]
Sanchez Navarro A, Martinez Cabarga M, Dominguez-Gil Hurle A. Comparative study of the influence of Ca2+ on absorption parameters of ciprofloxacin and ofloxacin.
J Antimicrob Chemother 1994 Jul;34(1):119-125.
Abstract: A comparative study was undertaken to investigate the influence of calcium on the absorption of ofloxacin and ciprofloxacin. The presence of CaCO3 did not significantly lower the partition coefficients of either fluoroquinolone although values for ofloxacin were significantly higher than those for ciprofloxacin (P = 0.0085). In intestinal sacs, the presence of 5000 mg/L CaCO3 significantly reduced both the absorption constant and the fraction of absorbed dose of 2000 mg/L ciprofloxacin but not 2000 mg/L ofloxacin. When the same concentration of CaCO3 was introduced into the isolated intestinal segments of rats, the absorption of both 200 mg/L ofloxacin and 400 mg/L ciprofloxacin was reduced significantly from 49% and 35% respectively to approximately 30% in each case. Co-administration of 500 mg/L CaCO3 to healthy volunteers significantly reduced the urinary excretion of 250 mg/L ciprofloxacin but not 200 mg/L ofloxacin although neither the fraction of absorbed dose nor the half-lives were markedly affected. Calcium therefore shares the same propensity as other cations in impairing the absorption of ciprofloxacin but not ofloxacin.
Sastrasinh M, Weinberg JM, Humes HD. The effect of gentamicin on calcium uptake by renal mitochondria.
Life Sci. 1982 Jun 28;30(26):2309-2315.
Abstract: The effect of the nephrotoxic aminoglycoside antibiotic, gentamicin, on calcium uptake by renal cortical mitochondria was assessed in vitro. Gentamicin was found to be a competitive inhibitor of mitochondrial Ca++ uptake. This effect displayed a dose response with a Ki of 233 microM and occurred at gentamicin concentrations below those that inhibit mitochondrial electron transport. These results further demonstrate the potential for gentamicin to alter membrane function and thereby contribute to toxic cell injury via its interactions with divalent cations.
Schneider M, Valentine S, Clarke GM, Newman MA, Peacock J. Acute renal failure in cardiac surgical patients, potentiated by gentamicin and calcium.
Anaesth Intensive Care 1996 Dec;24(6):647-650.
Abstract: A retrospective study in coronary artery bypass graft patients was undertaken to assess the effect of gentamicin and a bypass prime with a high calcium on the incidence of renal failure. Patients who received both Haemaccel (polygeline, Hoechst Marion Roussel) (calcium concentration 6.25 mmol/l) in the bypass prime and gentamicin perioperatively had a higher incidence of renal failure compared with those who received only Haemaccel (P = 0.005), only gentamicin (P = 0.002) or neither (P = 0.0001). We suggest that the combination be avoided in this group of patients.
Shafer RB, Nuttall FQ. Calcium and folic acid absorption in patients taking anticonvulsant drugs.
J Clin Endocrinol Metab 1975 Dec;41(06):1125-1129.
Abstract: Calcium and folic acid absorption were studied in 28 adult male epileptics on chronic anticonvulsant therapy. In 16 patients on diphenylhydantoin alone, calcium absorption was abnormal in 9. In 12 patients on both diphenylhydantoin and phenobarbital, calcium absorption was abnormal in 3 patients. Folic acid (3H-PGA) absorption was normal in all but one patient, while serum folate (less than 6.4 ng/ml) was reduced in all patients. Hypocalcemia (less than 8.5 mg/100 ml) occurred in only 2 patients, while serum alkaline phosphatase was elevated in 7 patients. These findings support the proposal that rickets and osteomalacia reported in patients on chronic anticonvulsant therapy results from reduced calcium absorption. The effect of these drugs appears to be the acceleration of the metabolism of vitamin D and an increase in the excretion of polar metabolites. This may result in reduced levels of 25-hydroxycholecalciferol and 1,25-dihydroxycholecalciferol which are necessary for normal absorption of calcium. Since calcium absorption may be impaired secondary to a relative vitamin D deficiency, a supplemental increase in vitamin D intake by patients on anticonvulsant drugs is recommended.
Schneider DL, Barrett-Connor EL, Morton DJ. Thyroid hormone use and bone mineral density in elderly men.
Arch Intern Med 1995 Oct 9;155(18):2005-2007.
Abstract: BACKGROUND: Excessive thyroid hormone use reduces bone density in women. Thyroid hormone use is much less common in men, who also have less osteoporosis. We examined bone mineral density in a community-based sample of elderly men who reported long-term thyroid hormone use. METHODS: All 685 white men aged 50 to 98 years from a Southern California community who participated in a study of osteoporosis were examined. Medication use was validated. Height and weight were measured. Bone mineral density was measured at the ultradistal radius and midshaft radius using single photon absorptiometry and at the hip and lumbar spine using dual energy x-ray absorptiometry. RESULTS: Thirty-three men taking a mean thyroxine-equivalent dose of 130 micrograms daily for an average of 15.5 years were compared with 653 nonusers. There were no significant differences in bone density at any site between users and nonusers, before or after controlling for age, body mass index, smoking, thiazide diuretics, and oral corticosteroid use. Bone density also did not differ according to thyroid hormone type, duration of use, or use of suppressive dose adjusted for body weight. CONCLUSIONS: Long-term thyroid hormone use was not associated with adverse effects on bone mineral density in men.
Spencer H, Kramer L. Antacid-induced calcium loss. Arch Intern Med 1983;143:657-658. (Editorial)
Threlkeld DS, ed. Blood Modifiers, Anticoagulants, Heparin. In: Facts and Comparisons Drug Information. St. Louis, MO: Facts and Comparisons, Jun 1997.
Threlkeld DS, ed. Hormones, Thyroid Hormones. In: Facts and Comparisons Drug Information. St. Louis, MO: Facts and Comparisons, Jun 1991.
Trovato A, Nuhlicek DN, Midtling JE. Drug-nutrient interactions. Am Fam Physician 1991 Nov;44(5):1651-1658. (Review)
Valdivieso A, Mardones JM, Loyola MS, Cubillos AM. [Hypomagnesemia associated with hypokalemia, hyponatremia and metabolic alkalosis. Possible complication of gentamycin therapy].
Rev Med Chil. 1992 Aug;120(8):914-919. [Article in Spanish]
Abstract: Hypomagnesemia is a serious abnormality with different causes and usually associated to other disorders of electrolyte metabolism. We report a female patient developing hypomagnesemia after administration of gentamycin. This was associated to severe hypokalemia, hyponatremia and metabolic alkalosis. Possible pathogenetic mechanisms and therapeutic measures are discussed.
Vernillo AT, Rifkin BR. Effects of tetracyclines on bone metabolism. Adv Dent Res 1998 Nov;12(2):56-62.
Abstract: The anti-resorptive properties of tetracyclines (TCs) and their non-antimicrobial, chemically modified analogues (CMTs) have enormous therapeutic potential in medicine and dentistry. Osseous destructive diseases associated with excessive mammalian collagenase (matrix metalloproteinase) activity and collagen breakdown include malignancy, arthritis, and periodontitis. However, apart from the significant antimatrix metalloproteinase effects of TCs, TCs/CMTs are also potent inhibitors of osteoclast function (i.e., anti-resorptive). Thus, TCs can affect several parameters of osteoclast function and consequently inhibit bone resorption by (1) altering intracellular calcium concentration and interacting with the putative calcium receptor; (2) decreasing ruffled border area; (3) diminishing acid production; (4) diminishing the secretion of lysosomal cysteine proteinases (cathepsins); (5) inducing cell retraction by affecting podosomes; (6) inhibiting osteoclast gelatinase activity; (7) selectively inhibiting osteoclast ontogeny or development; and (8) inducing apoptosis or programmed cell death of osteoclasts. TCs/CMTs, as anti-resorptive drugs, may act similarly to bisphosphonates and primarily affect osteoclast function.
Wahl TO, Gobuty AH, Lukert BP. Long-term anticonvulsant therapy and intestinal calcium absorption.
Clin Pharmacol Ther. 1981 Oct;30(4):506-512.
Abstract: Twelve patients on anticonvulsant therapy were studied to determine whether or not the drugs induced alterations in gastrointestinal absorption of calcium, response to parathyroid hormone (PTH), or serum 25-hydroxy vitamin D (25-OHD) concentrations. Fractional calcium absorption (FCaA) was determined by giving 45Ca intravenously and orally. The short-term response to PTH was assessed by giving 200 U of parathyroid extract (PTE) intravenously over 15 min and measuring hourly urine cyclic adenosine monophosphate (cAMP) and tubular reabsorption of phosphate (TRP). Calcemic response to PTH was followed by giving intramuscular injections of PTE, 200 U every 6 hr. FCaA was 30.8 +/- 3.7% lower than the normal of 42.2 +/- 2.5% (P less than 0.025), and baseline 25-OHD levels were 30.5 +/- 3.4 ng/ml (normal 15 to 50 ng/ml). Anticonvulsant drugs did not alter renal response to PTE. There was a rise in urinary cAMP from 3.7 +/- 0.23 to 6.1 +/- 0.47 mumol/gm creatinine (P less than 0.005) with a fall in TRP from 87.8 +/- 1.2% to 78.8 +/- 1.6% (P less than 0.005). Serum calcium rose from 9.4 +/- 0.1 to 11.1 +/- 0.3 mg/dl (P less than 0.005). We conclude that FCaA is low in patients receiving anticonvulsant drugs, even when serum 25-OHD levels and the response of bone and kidney to PTH remain normal.
Walker JA, Sherman RA, Cody RP. The effect of oral bases on enteral aluminum absorption.
Arch Intern Med 1990 Oct;150(10):2037-2039.
Abstract: Physicochemical considerations suggest that citrate may potentiate gastrointestinal aluminum absorption via the formation of an aluminum citrate moiety. We tested this hypothesis and also studied whether sodium bicarbonate would have a similar effect. Eight healthy adults each partook of four oral regimens: aluminum alone, aluminum plus sodium bicarbonate, aluminum plus citrate (as Shohl's solution), and citrate alone. Twenty-four hour urine collections were obtained immediately preceding and during the second day of each medication period for determination of aluminum content. A significant but similar increment in urinary aluminum occurred with both aluminum alone and aluminum plus sodium bicarbonate, while only a small increment was noted with Shohl's solution alone. The rise in urinary aluminum obtained with aluminum plus Shohl's solution, however, was nearly eight times that seen with either aluminum alone or aluminum plus sodium bicarbonate (327 micrograms vs 45 micrograms and 41 micrograms, respectively). Citrate thus appears to augment gastrointestinal aluminum absorption markedly, an effect not shared by an equivalent dose of sodium bicarbonate. Citrate administration to patients with renal failure who are also taking aluminum-containing medication may be harmful.
Watkins DW, Khalafi R, Cassidy MM, Vahouny GV. Alterations in calcium, magnesium, iron, and zinc metabolism by dietary cholestyramine.
Dig Dis Sci 1985 May;30(5):477-482.
Abstract: Cholestyramine is an effective drug for the reduction of plasma cholesterol because of its ability to sequester intestinal bile acids. Since metabolic alterations, including diminished intestinal absorption of vitamin D and osteomalacia have been reported with long-term use of this resin, the influence of cholestyramine on dietary balance of four mineral elements has been investigated. Wistar-strain rats were fed either a 2% cholestyramine or control diet for one month. Dietary intakes and fecal and urinary excretions of calcium, magnesium, iron, and zinc were determined using atomic absorption spectrophotometry during three, 3-day balance periods. Cholestyramine-fed rats had a net negative balance for calcium and a lower net positive balance for magnesium, iron, and zinc than the controls. Other effects of cholestyramine were an increased urinary excretion of calcium and magnesium, a decreased urinary zinc, and an alkalinization of urine. Blood and tissue cation content was unchanged except for a reduction in serum magnesium with resin feeding. Alterations in calcium, magnesium, and zinc metabolism might be explained by inadequate vitamin D absorption from the intestine followed by an increased secretion of parathyroid hormone. A diminished iron absorption due to resin binding could account for the reported disturbance in iron balance.
Weberg R, Berstad A. Gastrointestinal absorption of aluminum from single doses of aluminum containing antacids in man.
Eur J Clin Invest 1986 Oct;16(5):428-432.
Abstract: Ten subjects with normal renal function were given different single doses of aluminium containing antacids (1, 4, or 8 tablets). The antacid tablets (aluminium content 244 mg tablet-1) were chewed and swallowed either with water, with orange juice, or with citric acid solution. There was a marked increase in serum concentration of aluminium when the antacids was ingested with citric acid (P less than 0.001) or with orange juice (P less than 0.05). When antacids were taken with water, a slight, but significant increase in serum aluminium concentration was seen with 4, but not with 1 or with 8 tablets. Following all doses of antacid, a significant increase in 24 h urinary excretion of aluminium was seen. The estimated absorption of aluminium was 8 and 50 times higher when antacids were taken with orange juice or with citric acid, respectively, than when taken with water. Thus, measurable quantities of aluminium are absorbed from single oral doses of antacids. The absorption is substantially enhanced by concomitant ingestion of citric acid.
Werbach MR. Foundations of Nutritional Medicine. Tarzana, CA: Third Line Press, 1997. (Review).
Wise PH, Hall AJ. Heparin-induced osteopenia in pregnancy. Br Med J 1980 Jul 12;281(6233):110-111.
Abstract: Multiple vertebral compression fractures occurred in a pregnant woman receiving heparin over nine months. This phenomenon may be more common than is clinically recognised and warrants careful re-examination of the indications and method of administration of anti-coagulants during pregnancy.
Yeh JK, Aloia JF, Semla HM. Interrelation of cortisone and 1,25 dihydroxycholecalciferol on intestinal calcium and phosphate absorption. Calcif Tissue Int
1984 Sep;36(5):608-614.
Abstract: The interrelation of glucocorticoids and 1,25 dihydroxycholecalciferol (1,25(OH)2D3) on intestinal calcium and phosphate absorption was investigated. The active and passive transport of calcium and phosphate was evaluated by the in situ intestinal loop technique. Administration of cortisone resulted in a decrease of the luminal fluid and an increase of the luminal calcium and phosphate concentration. Under active transport conditions, administration of cortisone resulted in a decrease of net calcium absorption through two mechanisms: (1) depressed vitamin D-dependent calcium absorption, (2) increased vitamin D-independent calcium backflux. The enhancement of bidirectional phosphate flux by cortisone was independent of 1,25(OH)2D3. An enhancement of water movement by cortisone resulted in an increase of luminal calcium and phosphate concentration which favors the passive diffusion of these ions. Enhanced calcium diffusion by cortisone compensates for the inhibitory effect of cortisone on vitamin D-dependent calcium transport. However, enhanced phosphate diffusion by cortisone is additive to the effect of 1,25(OH)2D3.