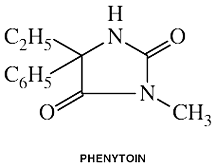
Phenytoin
Brand Names: Dilantin, Diphenylhydantoin
Clinical Names: Phenytoin
Summary
generic name: Phenytoin
trade names: Dilantin®, Diphenylhydantoin®
type of drug: Anticonvulsant.
used to treat: Seizure disorders, especially refractory partial epilepsy; also used in the treatment and prevention of seizures occurring during or following
neurosurgery.
overview of interactions:
• nutrient affected by drug: Folic Acid
• nutrient affected by drug: Vitamin B1
(Thiamine)
• multiple interactions: Vitamin B6
(Pyridoxine)
• nutrient affected by drug: Vitamin D
• nutrient affected by drug: Vitamin E
• nutrient affected by drug: Vitamin K
• nutrient affected by drug: Calcium
• nutrient affecting drug performance: Calcium
Interactions
nutrient affected by drug: Folic Acid
• adverse drug effects: Nearly all anticonvulsant drugs are known to have a marked impact on levels of folic acid in the body, especially through their effect of reducing folic acid absorption. This is especially important for women who are or might become pregnant. Phenytoin and related drugs are known to create a high degree of risk for birth defects in and of themselves. Furthermore, folic acid deficiency itself is highly associated with birth defects, especially neural tube defects, and supplementation of folic acid is generally agreed to provide significant benefit in protecting against these types of birth defects. The combination of these two factors creates an extraordinarily high degree of risk for women of child-bearing age who are using phenytoin.
• research: As with other anticonvulsants, most research shows that phenytoin (Dilantin) causes a decrease in plasma concentrations of folate in epileptic patients, most likely through depletion of total hepatic folate. Serum folate decreases when phenytoin therapy is initiated alone with no folate supplementation. Research on the use of folic acid by pregnant women using phenytoin has been positive in reducing the frequency and severity of birth defects among children born to women taking phenytoin. Even so, most physicians caution against such women becoming pregnant.
(Biale Y, Lewenthan H. Eur J Obstet Gynecol 1984 Nov;18(4):211-216; Ogawa Y, et al.
Epilepsy Res 1991 Jan-Feb;8(1):75-78; Yerby MS. Epilepsia 1987;28 Suppl 3:S29-36; Lewis DP, et al.
Ann Pharmacother 1998 Jul-Aug;32(7-8):802-817; Dansky LV, et al. Ann Neurol 1987 Feb;21(2):176-182; Nulman I, et al.
Drugs 1999 Apr;57(4):535-544; Shane-McWhorter L, et al. Pharmacotherapy 1998 Nov-Dec;18(6):1360-1364; Hendal J et al.
Acta Neurol Scand 1984;69:226-231; Roe DA. 1985, 249; Carl GF, et al. J Nutr
1997 Nov;127(11):2231-2238.)
• nutritional support: Women receiving folate-lowering drugs may be at increased risk of adverse pregnancy outcomes. Therefore, epileptic women contemplating pregnancy should be treated with the minimum number of folate-lowering drugs possible and receive folic acid supplementation. Several studies have used doses ranging from 0.1 to 5 mg/day with similar outcomes. Conservative practice would recommend that the prescribing physician monitor serum levels and titrate folic acid supplementation to these values. A dose of 4 - 5 mg/day was recommended by the CDC for women, not necessarily using anticonvulsants, who have had prior experiences with neural tube defects. In general, a daily supplemental dose of 400-800 mcg folate is probably adequate to compensate for any potential deficiency due to phenytoin. However, if pregnancy is anticipated, folic acid, at a daily dose of 5 mg, should be administered 3 months before conception and during the first trimester to prevent folic acid deficiency-induced malformations. Many nutritionally oriented healthcare professionals advise that every potentially pregnant woman should be taking folic acid.
(Nulman I, et al. Drugs 1999 Apr;57(4):535-544; Lewis DP, et al. Ann Pharmacother 1998 Jul-Aug;32(7-8):802-817; University of Maryland Drug Information Service.)
A number of anticonvulsants are enzyme-inducing agents and thereby increase the clearance of the oral contraceptive steroids. The use of birth control pills or Norplant does not reduce the need for folic acid because many antiepileptic drugs interfere with the efficacy of contraceptives. Studies have shown that the most commonly used antiepileptic medications, including phenytoin, induce the cytochrome P450 enzymes that metabolize synthetic estrogens (e.g., ethinyl estradiol and mestranol), causing a 40% reduction in serum levels. Thus it becomes even more imperative for a woman using phenytoin who wishes to become pregnant to add folic acid to her prenatal regimen.
(Nulman I, et al. Drugs 1999 Apr;57(4):535-544; Shane-McWhorter L, et al.
Pharmacotherapy 1998 Nov-Dec;18(6):1360-1364; Back DJ, Orme ML. Clin Pharmacokinet
1990 Jun;18(6):472-484; Crawford P, et al. Br J Clin Pharmacol 1990 Dec;30(6):892-896.)
• nutritional synergy: Berg et al reported that the addition of folic acid to phenytoin therapy produced changes in blood levels of phenytoin. Later in the same year, in their review of the literature on phenytoin-folate interactions, Lewis et al proposed that folate should always be prescribed with phenytoin because of a synergistic effect. Folic acid supplementation in folate-deficient patients with epilepsy changes the pharmacokinetics of phenytoin and may increase risk of possible seizure breakthrough by leading to lower serum phenytoin concentrations. Folate is hypothesized to be a cofactor in phenytoin metabolism and may be able to assist in obtaining a concentration where phenytoin appears to be at steady-state, but in reality, is not. Phenytoin and folic acid therapy initiated concomitantly prevents decreased folate and phenytoin obtains steady-state concentrations sooner. Thus, Lewis and his cohorts concluded that folic acid supplementation should be initiated each time phenytoin therapy commences because of the hypothesized cofactor mechanism, decreased adverse effects associated with folate deficiency, and better seizure control with no perturbation of phenytoin pharmacokinetics.
(Berg MJ, et al. J Am Diet Assoc 1995 Mar;95(3):352-356; Lewis DP, et al. Ann Pharmacother 1995 Jul-Aug;29(7-8):726-735.)
• adverse drug effects: Individuals using phenytoin suffer from an increased incidence of gingival hyperplasia (gum overgrowth). While regular dental and gum care by a dentist and/or periodontist can help prevent or reduce this phenomenon, it is prone to occur as long as phenytoin is taken. (Francetti L, Maggiore E, Marchesi A, et al.
Prev Assist Dent 1991;17(30):40-43; Fitchie JG, et al. Compendium 1989;10(6):314; Steinberg SC, Steinberg AD.
J Periodontol 1982; 53(7)429-433.) Seymour RA, et al. J Clin Periodontol
1996 Mar;23(3 Pt 1):165-175. McLaughlin WS, et al. J Clin Periodontol 1995 Dec;22(12):942-945.
• nutritional support: The topical application of folate has been found to be generally helpful in the treatment of gingival overgrowth. However, the level of dietary folate did not correlate with changes in hyperplasia in experimental subjects. Folate mouthwash appears to have an influence on gum health through local rather than systemic influence. Further research conducted with a focus on phenytoin-induced changes in the gums indicates that daily rinses with a folate-based mouthwash may also inhibit gum disease due to phenytoin.
(Pack AR. J Clin Periodontol 1986 Aug;13(7):671-676; Drew HJ, et al. J Clin Periodontol 1987 Jul;14(6):350-356.)
• nutrient affected by drug: Vitamin B1
(Thiamine)
• research: In a study of seventy-two epileptic patients receiving phenytoin alone or in combination with phenobarbital for more than four years, Botez et al found that 31% of the patients had subnormal blood thiamine levels and 30% had low folate. The vitamin deficiencies were independent phenomena. In their study of the effects of phenytoin on the in vivo kinetics of thiamine in rat nervous tissues Patrini et al reported that phenytoin appeared to interfere mainly with thiamine and thiamine monophosphate (TMP) uptake, thiamine pyrophosphate dephosphorylation (TPP) to TMP, and TPP turnover times, and that these effects were particularly prominent in the cerebellum and in the brainstem of chronically treated animals.
(Botez MI, et al. Epilepsy Res 1993 Oct;16(2):157-163; Botez MI, et al. Can J Neurol Sci
1982 Feb;9(1):37-39; Patrini C, et al. Brain Res 1993 Nov 19;628(1-2):179-186.
• nutritional support: In their six month-long clinical trial Botez and his cohorts found that thiamine (50 mg/day) enhanced function in both verbal and non-verbal IQ testing. In particular, higher scores were recorded on the block design, digit symbol, similarities and digit span subtests. The researchers concluded that, in epileptics chronically treated with phenytoin, thiamine improves neuropsychological functions, such as visuo-spatial analysis, visuo-motor speed and verbal abstracting ability. For individuals using phenytoin, supplementation with 50-100 mg per day of thiamine would seem prudent, especially given that the vitamin has no known toxicity. Even so, individuals using phenytoin should consultant with their prescribing physician and/or a nutritionally trained healthcare professional before introducing any significant levels of supplementation into their therapeutic regime.
(Botez MI, et al. Epilepsy Res 1993 Oct;16(2):157-163.)
multiple interactions: Vitamin B6
(Pyridoxine)
• nutrient affecting drug performance: There have been some reports that vitamin B6, supplemented in doses as low as 80 mg daily, may reduce the effectiveness of phenytoin by up to 50%.
(Hansson O, Sillanpaa M. Lancet 1976 Jan 31;1(7953):256.)
• nutrient affected by drug: Phenytoin can lower plasma levels of vitamin B6. Apart from its emerging reputation as a risk factor in cardiovascular disease homocysteine has been used as an experimental convulsant. Several researchers have found elevated levels of homocysteine in epileptics using anticonvulsant medications, but at this point evidence is mixed as to whether this is attributable to the underlying disease process, the medication, or both. Supplementation with vitamin B6, vitamin B12, or folate has been considered as offering therapeutic potential since nutritional deficiencies of these nutrients are associated with increased homocysteine plasma concentrations.
(Schwaninger M, et al. Epilepsia 1999 Mar;40(3):345-350.)
• nutritional support and concerns: Supplemental use of vitamin B6 at high dosages (200 mg or more per day) carries known risks, including eventual damage to sensory nerves. Individuals using phenytoin should consultant with their prescribing physician and/or a nutritionally trained healthcare professional before introducing supplemental vitamin B6 into their therapeutic regime.
nutrient affected by drug: Vitamin D
• research: Bone loss has been associated with phenytoin therapy and is considered to be due to phenytoin's interference with vitamin D metabolism. Phenytoin and other anticonvulsants are inducers of cytochrome P-450 and the mixed-function oxidase system. Thus, anticonvulsants impair mineralisation, leading to osteomalacia and osteoporosis. Long-term treatment can cause excessive metabolism and deficiency of vitamin D and is believed to be associated with decreased bone mineral density and bone loss. Usually lab tests will reveal elevated Alk Phos levels and mild hypocalcemia. After finding no pattern of low serum levels of vitamin D (25[OH]D) or radiologic evidence of osteomalacia or rickets in over 400 individuals using anticonvulsants in Florida, Williams et al concluded that the climate provided adequate exposure to sunshine and thereby prevented the development of anticonvulsant-induced osteomalacia or rickets.
(D'Erasmo E, et al. Recenti Prog Med 1998 Oct;89(10):529-533; Chung S, Ahn C.
Brain Dev 1994 Sep-Oct;16(5):382-385; Pluskiewicz W, Nowakowska J. Ultrasound Med Biol 1997;23(4):553-558; Bone HG.
JAMA 1983;249:939; Williams C, et al. Southern Med J 1984 Jul;77(7):834-836, 842; Livingston S.
JAMA 1983;249:939; Jones G, Sambrook PN. Drug Saf 1994 Jun;10(6):480-489.)
• nutritional support: A moderate dose of 400-1500 IU per day of Vitamin D could exert a protective function for individuals using phenytoin who are concerned about potential drug-induced rickets or osteoporosis. However, as suggested above, regular exposure to sunlight might provide adequate stimulation to enable the body to produce necessary levels of vitamin D. Others have advocated a proactive approach toward the risks of bone loss while voicing caution that given the increased risk of osteomalacia among those taking anticonvulsants, withdrawal from such drugs carries potential for increased risk of seizure-related fractures.
(Tomita S, et al. J Steroid Biochem Mol Biol 1991 Oct;39(4A):479-485; Christiansen C, et al.
Br Med J 1973 Dec 22;4(894):695-701; Macallan DC, et al. Postgrad Med J 1992 Feb;68(796):134-136; Duus BR.
Injury 1986 Jan;17(1):31-33; Bone HG. JAMA 1983;249:939.)
nutrient affected by drug: Vitamin E
• research: Phenytoin reduces serum levels of vitamin E.
(Sullivan, C, et al. Med J Austr 1990 Jun 4;152(11):613-614.)
• nutritional support: Supplementing with vitamin E has been reported by some researchers to reduce the incidence of seizures, most likely due to its antioxidant activities. However, others have not been able to confirm these findings of independent anticonvulsant effects from vitamin E. Other clinical studies have found vitamin E (d-alpha-tocopherol) to be a useful adjunct to anticonvulsant drugs. Improvement has been reported even in patients with complex partial seizures, which are often resistant to drug therapy.
(Wiehl W, Hart LL. DICP 1991 Apr;25(4):362-363; Sullivan, C, et al. Med J Austr 1990 Jun 4;152(11):613-614; Raju GB, et al.
Epilepsia 1994 Mar-Apr;35(2):368-732.)
nutrient affected by drug: Vitamin K
• research: Phenytoin interferes with vitamin K metabolism as indicated by a raised serum osteocalcin level. In regard to pregnancy, the use of phenytoin has been associated with vitamin K deficiencies in newborns. Children born of mothers using phenytoin have been known to suffer from calcification problems and one study with rats has indicated a role in birth defects involving excessive calcification, such as represented by Binder's Syndrome.
(Keith DA, et al. Clin Pharmacol Ther 1983 Oct;34(4):529-532; Keith DA, et al. Med Hypotheses 1979 Dec;5(12):1347-1351; Cornelissen M, et al.
Am J Obstet Gynecol 1993 Mar;168(3 Pt 1):923-928; Howe AM, et al. Aust Dent J 1992 Dec;37(6):453-460.)
• nutritional support: At this point research has produced no consensus as to appropriate therapeutic dose of Vitamin K to counter the adverse effects of phenytoin. Increasing the amount of green leafy vegetables in the diet could provide multiple benefits, in addition to enhancing Vitamin K levels. Further supplementation with 1 mg Vitamin K1 daily would also be prudent for most individuals taking phenytoin. Among pregnant women taking anticonvulsants, research indicates that a higher dose of Vitamin K1, 10 mg per day, can prevent vitamin K deficiency in their infants.
interaction: Calcium
• nutrient affected by drug: Dilantin adversely affects calcium metabolism through its interference with vitamin D metabolism.
• nutrient affecting drug performance: Calcium is commonly used in conjunction with vitamin D to protect bone integrity. However, administration of phenytoin with calcium preparations should be separated by at least three hours to prevent a decrease in phenytoin absorption.


Please read the disclaimer concerning the intent
and limitations of the information provided here.
Do not rely solely on the information in this article.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
References
Back DJ, Orme ML. Pharmacokinetic drug interactions with oral contraceptives.
Clin Pharmacokinet 1990 Jun;18(6):472-484.
Abstract: Oral contraceptive steroids are used by an estimated 60 to 70 million women world-wide. Over the past 20 years there have been both case reports and clinical studies on the topic of drug interactions with these agents. Some of the interactions are of definite therapeutic relevance, whereas others can be discounted as being of no clinical significance. Pharmacological interactions between oral contraceptive steroids and other compounds may be of 2 kinds: (a) drugs may impair the efficacy of oral contraceptive steroids, leading to breakthrough bleeding and pregnancy (in a few cases, the activity of the contraceptive is enhanced); (b) oral contraceptive steroids may interfere with the metabolism of other drugs. A number of anticonvulsants (phenobarbital, phenytoin, carbamazepine) are enzyme-inducing agents and thereby increase the clearance of the oral contraceptive steroids. Valproic acid has no enzyme-inducing properties, and thus women on this anticonvulsant can rely on their low dose oral contraceptive steroids for contraceptive protection. Researchers are now beginning to unravel the molecular basis of this interaction, with evidence of specific forms of cytochrome P450 (P450IIC and IIIA gene families) being induced by phenobarbital. Rifampicin, the antituberculous drug, also induces a cytochrome P450 which is a product of the P450IIIA gene subfamily. This isozyme is one of the major forms involved in 2-hydroxylation of ethinylestradiol. Broad spectrum antibiotics have been implicated in causing pill failure; case reports document the interaction, and general practitioners are convinced that it is real. The problem remains that there is still no firm clinical pharmacokinetic evidence which indicates that blood concentrations of oral contraceptive steroids are altered by antibiotics. However, perhaps this should not be a surprise, given that the incidence of the interaction may be very low. It is suggested that an individual at risk will have a low bioavailability of ethinylestradiol, a large enterohepatic recirculation and gut flora particularly susceptible to the antibiotic being used. Two drugs, ascorbic acid (vitamin C) and paracetamol (acetaminophen), give rise to increased blood concentrations of ethinylestradiol due to competition for sulphation. The interactions could have some significance to women on oral contraceptive steroids who regularly take high doses of either drug. Although on theoretical grounds adsorbents (e.g. magnesium trisilicate, aLuminium hydroxide, activated charcoal and kaolin) could be expected to interfere with oral contraceptive efficacy, there is no firm evidence that this is the case. Similarly, there is no evidence that smoking alters the pharmacokinetics of oral contraceptive steroids. These agents are now well documented as being able to alter the pharmacokinetics of other concomitantly administered drugs.
Berg MJ, Fincham RW, Ebert BE, Schottelius DD. Decrease of serum folates in healthy male volunteers taking
phenytoin. Epilepsia 1988 Jan-Feb;29(1):67-73.
Abstract: The effect of phenytoin (PHT) on serum folate and the effect of additional oral folic acid (FA) on serum folate during continued treatment with PHT were studied in 13 healthy male subjects 20-35 years of age. The study was divided into two phases: Phase I determined Vmax (mg/kg/day) and Km (microgram/ml) of PHT in order to calculate the PHT doses needed for the second phase. Phase II was a four-way cross-over study to examine the effect of 1 and 5 mg FA on total serum PHT concentrations 1 microgram/ml less and 5 micrograms/ml greater than the subject's Km, Km-1 and Km+5, respectively. Both phases examined the effect of PHT on serum folate. In Phase I, serum folate decreased by a mean and standard deviation of 42.15 +/- 21.44% after an average of 24.15 +/- 5.63 days of PHT administration, with a mean steady-state total serum PHT concentration of 8.45 +/- 2.70 micrograms/ml. Mean percentage decreases in serum folate before the addition of 1 and 5 mg FA in Phase II were 12.80 +/- 31.45% and 23.24 +/- 21.24% for Km-1 and Km+5, respectively. The average numbers of days of PHT administration and total serum PHT concentrations before FA administration were 9.52 +/- 3.34 and 15.84 +/- 7.02 days, and 2.60 +/- 2.18 and 8.64 +/- 3.44 micrograms/ml, for Km-1 and Km+5, respectively.
Berg MJ, Stumbo PH, Chenard CA, et al. Folic acid improves phenytoin
pharmacokinetics. J Am Diet Assoc 1995 Mar;95(3):352-356.
Abstract: Phenytoin (PHT) therapy to control seizures decreases serum folate levels in half of epileptic patients, thus increasing the risk of folate depletion. Supplementation with folic acid prevents deficiency but also changes PHT pharmacokinetics. Kinetic monitoring of PHT when folic acid is provided as a supplement has not been reported in women of child-bearing age. This study of six fertile women examined the interdependence of PHT and folic acid in a randomized crossover study of two treatments: treatment 1 consisted of 300 mg sodium PHT per day and treatment 2 consisted of 300 mg sodium PHT plus 1 mg folic acid per day. Dietary folic acid intake was calculated daily. During treatment 1, serum folate level decreased 38.0 +/- 18.6% (mean +/- standard deviation) and serum PHT concentration was in the low therapeutic range (43.92 +/- 14.52 mumol/L). During treatment 2, serum folate level increased 26.0 +/- 33.4%, and serum PHT level (39.04 +/- 14.16 mumol/L) was similar to that in treatment 1. Only one subject attained PHT steady state during treatment 1, but four subjects achieved steady state during treatment 2. Dietary folate intakes during treatments 1 and 2 were not significantly different. This study suggests an interdependence between PHT and folic acid and supports the observation that fertile women treated with PHT require folic acid supplementation to maintain a normal serum folate level.
Biale Y, Lewenthan H. Effect of folic acid supplementation on congenital malformations due to anticonvulsive drugs.
Eur J Obstet Gynecol 1984 Nov;18(4):211-216.
Abstract: A study was conducted to determine the frequency of malformations among newborn infants of mothers receiving anticonvulsive therapy, with and without supplementation of folic acid. In the retrospective part of the study, the frequency of congenital malformations among the 66 newborn of 24 women who received anticonvulsive drugs without the supplementation of folic acid was 15% (10 children). The defects noted were congenital heart disease, cleft lip and palate, neural tube defects and skeletal abnormalities. Three out of the 10 children were stillborn or died immediately after delivery. In the prospective study of the 22 epileptic women with folic acid supplementation to their anticonvulsive regimen, 33 infants were born alive, without congenital malformations and of normal body weight. The teratogenic activity of anticonvulsant drugs seems to be mediated by interference with folic acid metabolism, and such activity might be influenced by hereditary and environmental factors. When an epileptic woman wishes to become pregnant, it is recommended that folic acid be added to her regimen.
Bone HG. Long-term anticonvulsant therapy and vitamin D metabolism. JAMA 1983;249:939. (Review)
Botez MI, Joyal C, Maag U, Bachevalier J. Cerebrospinal fluid and blood thiamine concentrations in phenytoin-treated epileptics.
Can J Neurol Sci 1982 Feb;9(1):37-39.
Abstract: Thiamine and folate levels in blood and cerebrospinal fluid (CSF) were determined by microbiological assays in 23 control subjects and 11 phenytoin-treated epileptics. There was no significant difference between the two groups for serum and CSF folate levels. There was, however, a statistically significant difference between the groups for both whole blood thiamine and CSF thiamine levels. Epileptic patients being treated with phenytoin had lower values than control subjects.
Botez MI, Botez T, Ross-Chouinard A, Lalonde R. Thiamine and folate treatment of chronic epileptic patients: a controlled study with the Wechsler IQ scale. Epilepsy Res 1993 Oct;16(2):157-163.
Abstract: Seventy-two epileptic patients receiving phenytoin (PHT) alone or in combination with phenobarbital for more than 4 years were divided into four groups, the first taking two placebo tablets per day; the second folate (5 mg/day) and placebo; the third placebo and thiamine (50 mg/day); and the fourth both vitamins. The clinical trial lasted 6 months. At baseline assessment, 31% of the patients had subnormal blood thiamine levels and 30% had low folate. The vitamin deficiencies were independent phenomena. It was found that thiamine improved neuropsychological functions in both verbal and non-verbal IQ testing. In particular, higher scores were recorded on the block design, digit symbol, similarities and digit span subtests. Folate treatment was ineffective. These results indicate that, in epileptics chronically treated with PHT, thiamine improves neuropsychological functions, such as visuo-spatial analysis, visuo-motor speed and verbal abstracting ability.
Carl GF, Hudson FZ, McGuire BS Jr. Phenytoin-induced depletion of folate in rats originates in liver and involves a mechanism that does not discriminate folate form.
J Nutr 1997 Nov;127(11):2231-2238.
Chung S, Ahn C. Effects of anti-epileptic drug therapy on bone mineral density in ambulatory epileptic children. Brain Dev 1994 Sep-Oct;16(5):382-385.
Abstract: In order to assess the bone changes in the subjects receiving anti-epileptic drugs (AEDs), bone mineral densities (BMDs) of the arms, legs, ribs, pelvis, spine, and the whole body were scanned in 78 epileptic children and in 78 controls using dual photon absorptiometry. The study subjects were classified according to the duration of the monotherapy with phenobarbital (PB) or phenytoin (PHT); those who received AEDs for less than 12 months as Group I, for 13-23 months as Group II, and for 24 months as Group III. Group III was subclassified according to the kind of AEDs administered, into those receiving PB as Group IIIp, and those receiving PHT as Group IIId. There was no significant differences in the BMDs of each area, when compared to each control in Groups I and II. In Group III, there were significant differences in ribs and spine, according to the duration of administration. In Group IIIp, there was a significant difference in ribs and spine, and, in Group IIId, there was a significant difference in most of the areas. These results show that the measurement of BMDs in the ribs and spine is necessary for the early detection of subtle bone loss, and it is recommended that vitamin D be administered to children with epilepsy receiving AEDs over 24 months.
Collins CS, Bailey LB, Hillier S, Cerda JJ, Wilder BJ. Red blood cell uptake of supplemental folate in patients on anticonvulsant drug therapy. Am J Clin Nutr 1988 Dec;48(6):1445-1450.
Abstract: A group of epileptics (n = 18) and a control group (n = 10) of subjects aged 21-42 y were given 1-mg supplements of folate daily for 1 mo. Anticonvulsant therapy involved phenytoin alone or in combination with phenobarbital. Serum and red blood cell (RBC) folate levels were determined on days 1, 14, and 28. Mean serum and RBC folate levels were greater (p less than 0.05) for the control subjects compared with the epileptic subjects throughout the study. The percent increase in either serum or RBC folate was not different (p greater than 0.05) between the groups. The percent increase in serum folate when expressed as a percent of RBC folate was greater (p less than 0.05) for those epileptics who initially had deficient blood folate levels (serum folate less than 7 nmol/L; RBC folate less than 317 nmol/L) than those who did not. Deficient epileptics may have had an impaired RBC incorporation of circulating (serum) folate compared with nondeficient epileptics.
Collins N, Maher J, Cole M, Baker M, Callaghan N. A prospective study to evaluate the dose of vitamin D required to correct low 25-hydroxyvitamin D levels, calcium, and alkaline phosphatase in patients at risk of developing antiepileptic drug-induced
osteomalacia. Q J Med 1991 Feb;78(286):113-122.
Abstract: The dose of vitamin D3 required to maintain normal serum 25-hydroxyvitamin D levels in epileptic patients was evaluated in a prospective study. Patients were divided into two groups, comprising 14 institutionalized and 18 non-institutionalized subjects; they were taking carbamazepine, phenytoin and phenobarbitone, alone or in combination. The study was divided into a dose titration stage and a further period of assessment on a fixed dose after attainment of normal serum 25-hydroxyvitamin D levels. Seventeen of the 18 non-institutionalized patients achieved normal levels over a period of 12 months; the remaining patient became normal after 15 months. The dose required to achieve normal levels ranged from 400 to 4000 IU/day; three patients required less than 2400 IU vitamin D3, 12 required 2400 IU and three required greater than 2400 IU. All institutionalized patients achieved normal levels over a period of 12 months; six patients required less than 2400 IU, six required 2400 IU and two required greater than 2400 IU vitamin D3. Raised alkaline phosphatase levels occurred in 11 patients, and reverted to normal in six patients during the initial return of 25-hydroxyvitamin D levels to normal. During the second 12 months, when patients were taking a fixed dose of vitamin D3, alkaline phosphatase increased in five patients who had achieved normal levels. During this phase normal 25-hydroxyvitamin D levels were not maintained in five patients. There was a significant seasonal variation of 25-hydroxyvitamin D levels institutionalized patients, being highest in June and lowest in December. Our findings show that while there was a wide range in the dose required to achieve normal serum 25-hydroxyvitamin D levels--between 400 and 4000 IU/day--78 per cent of patients responded to a dose of 2400 IU/day.
Cornelissen M, Steegers-Theunissen R, Kollee L, Eskes T, Vogels-Mentink G, Motohara K, De Abreu R, Monnens L. Increased incidence of neonatal vitamin K deficiency resulting from maternal anticonvulsant therapy.
Am J Obstet Gynecol 1993 Mar;168(3 Pt 1):923-928.
Abstract: OBJECTIVE: The null hypothesis of our study is that the incidence of vitamin K deficiency in mother-infant pairs exposed to anticonvulsant drugs is not higher than in controls. STUDY DESIGN: In this multicenter observational case-control study, 25 pregnant women receiving anticonvulsant therapy and 25 pregnant controls were studied for PIVKA-II (protein induced by vitamin K absence of factor II) and vitamin K1 concentrations at 32 weeks' gestation and at delivery. RESULTS: PIVKA-II was detectable in 54% of cord samples of the anticonvulsant group and in 20% of controls (chi 2, p = 0.01). In both groups vitamin K1 cord blood levels were predominantly below the detection limit. Maternal vitamin K1 concentrations were lower in women with epilepsy than in controls (Wilcoxon's rank sum test, p < 0.05), but PIVKA-II was rarely present. CONCLUSIONS: The incidence of vitamin K deficiency is increased in neonates exposed to anticonvulsant drugs prenatally. Their mothers, however, are rarely vitamin K deficient.
Cornelissen M, Steegers-Theunissen R, Kollee L, Eskes T, Motohara K, Monnens L. Supplementation of vitamin K in pregnant women receiving anticonvulsant therapy prevents neonatal vitamin K deficiency.
Am J Obstet Gynecol 1993 Mar;168(3 Pt 1):884-888.
Abstract: OBJECTIVE: The null hypothesis of this study is that extra vitamin K administered to pregnant women on a regimen of enzyme-inducing anticonvulsant therapy will not decrease the frequency of symptoms of vitamin K deficiency in their neonates. STUDY DESIGN: A multicenter case-control study was performed on 16 pregnant women on anticonvulsant therapy who received 10 mg of vitamin K1 daily from 36 weeks of pregnancy onward. Concentrations of PIVKA-II (protein induced by vitamin K absence for factor II) and of vitamin K1 were determined in cord blood and compared with those in 20 controls. RESULTS: In none of 17 cord samples was PIVKA-II detectable, compared with 13 of 20 in controls (chi 2, p < 0.001). Median cord vitamin K1 level was 530 pg/ml compared with below detection limit in most controls. CONCLUSIONS: Antenatal vitamin K1 treatment decreases the frequency of vitamin K deficiency in neonates of mothers on anticonvulsant therapy.
Crawford P, Chadwick DJ, Martin C, Tjia J, Back DJ, Orme M. The interaction of phenytoin and carbamazepine with combined oral contraceptive steroids.
Br J Clin Pharmacol 1990 Dec;30(6):892-896.
Abstract: Patients taking oral contraceptive steroids (OCS) are known to suffer contraceptive failure while taking anticonvulsants such as phenobarbitone, phenytoin and carbamazepine. We have studied the single dose kinetics of ethinyloestradiol (EE2); 50 micrograms, and levonorgestrel (Ng); 250 micrograms in groups of women before and 8-12 weeks after starting therapy with phenytoin (n = 6) and carbamazepine (n = 4). The area under the plasma concentration-time curve (AUC) was measured over a 24 h period for each steroid and significant reductions were seen with both anticonvulsants. Phenytoin reduced the AUC for EE2 from 806 +/- 50 (mean +/- s.d.) to 411 +/- 132 pg ml-1 h (P less than 0.05) and for Ng from 33.6 +/- 7.8 to 19.5 +/- 3.8 ng ml-1 h (P less than 0.05). Carbamazepine reduced the AUC for EE2 from 1163 +/- 466 to 672 +/- 211 pg ml-1 h (P less than 0.05) and for Ng from 22.9 +/- 9.4 to 13.8 +/- 5.8 ng ml-1 h (P less than 0.05). These changes are compatible with the known enzyme inducing effects of phenytoin and carbamazepine. Patients taking these anticonvulsants will need to be given increased doses of OCS (equivalent to 50-100 micrograms EE2 daily) to achieve adequate contraceptive effects.
Dansky LV, Andermann E, Rosenblatt D, Sherwin AL, Andermann F. Anticonvulsants, folate levels, and pregnancy outcome: a prospective study. Ann Neurol 1987 Feb;21(2):176-182.
Abstract: Folate levels in serum and red cells, as determined by a microbiological assay using Lactobacillus casei, and plasma anticonvulsant concentrations were monitored concurrently in nonpregnant (50 subjects) and pregnant (49 pregnancies in 46 subjects) epileptic women. Twenty-three (46%) nonpregnant women had subnormal serum folate levels and 4 nonpregnant women (8%) showed subnormal red cell folate levels. In pregnant women not taking folate supplements, the incidence of folate deficiency increased as the pregnancy advanced. Pregnant women taking folate supplements achieved normal or supranormal blood folate concentrations. In both nonpregnant and pregnant women, serum and red cell folate levels were inversely correlated with plasma concentrations of phenytoin and of phenobarbital, and with the number of anticonvulsants. In 49 pregnancies, there were 10 abnormal outcomes (20.4%): 4 spontaneous abortions (8.2%) and 6 children with major congenital malformations (12.2%). Blood folate levels were significantly lower in pregnancies with an abnormal outcome than in those with a normal outcome. The results suggest a dose-response relationship among anticonvulsants, folate, and adverse pregnancy outcome.
Drew HJ, Vogel RI, Molofsky W, Baker H, Frank O. Effect of folate on phenytoin
hyperplasia. J Clin Periodontol 1987 Jul;14(6):350-356.
Abstract: There have been some reports that folic acid inhibits phenytoin-induced gingival hyperplasia. The purpose of this double-blind study was to quantify clinically the effects of both systemic and topical administration of folic acid on phenytoin-induced gingival overgrowth in man. For a period of 6 months, one group of phenytoin patients received 2 daily topical applications of a folate solution. An additional group received 2 daily doses of systemic folate while a control group received placebo medication. Results indicate that throughout the 180-day period of the study, the topical folate significantly inhibited gingival hyperplasia to a greater extent than either systemic folate or placebo groups.
Fitchie JG, Comer RW, Hanes PJ, Reeves GW. The reduction of phenytoin-induced gingival overgrowth in a severely disabled patient: a case report.
Compendium 1989 Jun;10(6):314, 317-20.
Abstract: Gingival overgrowth frequently occurs in patients medicated with phenytoin (5,5-diphenylhydantoin) to control epileptic seizures. In a recent study, gingival overgrowth was observed in a patient in an experimental group evaluating an automatic toothbrushing system for severely disabled patients. During the evaluation period, which included an oral hygiene regimen provided by an attendant or housemate and a regimen with the Mississippi Dental Care System (MDCS), the patient's gingival overgrowth was noticeably reduced. The results of this study indicate that control of local factors with the MDCS is significantly better than the routine home care regimen, and that the phenytoin-associated gingival overgrowth in this patient was reduced by MDCS.
Francetti L, Maggiore E, Marchesi A, Ronchi G, Romeo E. Oral hygiene in subjects treated with diphenylhydantoin: effects of a professional program.
Prev Assist Dent 1991 May-Jun;17(3):40-43. [Article in Italian]
Abstract: The purpose of the present study was to investigate, on a longitudinal basis, the effectiveness of a specific preventive dental program for patients who are taking phenytoin for seizure control. The results confirm that a preventive dental program, consisting on frequent prophylaxis and plaque control, is effective in minimizing clinically gingival enlargement associated with phenytoin therapy, even in patients who present histological aspects of gingival hyperplasia.
Hansson O, Sillanpaa M. Pyridoxine and serum concentration of phenytoin and
phenobarbitone. Lancet 1976 Jan 31;1(7953):256. (Letter)
Hathcock JN. Metabolic mechanisms of drug-nutrient interactions. Fed Proc 1985 Jan;44(1 Pt 1):124-129.
Abstract: Metabolic mechanisms of nutrition and drug interactions include 1) the effects of diet on drug metabolism and action and 2) the effects of drugs on nutritional processes. The type, amount, and timing of foods consumed influence drug dissolution, absorption, distribution, metabolism, and excretion. High-fat meals enhance the absorption of griseofulvin and some other drugs. Milk and other sources of calcium inhibit absorption of tetracycline. High-fat meals increase plasma concentrations of free fatty acids and thereby displace many drugs from binding sites on plasma albumin. High-protein diets increase the activity of the mixed-function oxidase system and enhance the metabolism of numerous drugs. High-electrolyte intakes increase excretion of lithium and also diminish the action of diuretic agents. Bile acid sequestrants and some laxatives decrease lipid digestion and absorption, as well as absorption of the fat-soluble vitamins. Numerous drugs, including tetracycline and cholestyramine, bind iron and decrease its absorption. Coumarins inhibit the function of vitamin K. Phenobarbital and other anticonvulsants are inducers of cytochrome P-450 and the mixed-function oxidase system. Long-term treatment with these inducers can cause excessive metabolism and deficiency of vitamin D. Prooxidant drugs such as chloroquine, drugs detoxified by conjugation with glutathione, and alcohol can deplete reduced glutathione with consequent effects on amino acid transport and the redox status of cells. Acid-forming foods acidify the urine and increase the loss of alkaline drugs such as the amphetamines. Base-forming drugs increase the loss of acidic drugs such as barbiturates. The range of metabolic interactions of drugs and nutrients includes the full scope of physiological processes to which drugs and nutrients are subject.
Hiilesmaa VK, Teramo K, Granstrom ML, Bardy AH. Serum folate concentrations during pregnancy in women with epilepsy: relation to antiepileptic drug concentrations, number of seizures, and fetal outcome. Br Med J (Clin Res Ed) 1983 Aug 27;287(6392):577-579.
Abstract: Serum folate concentrations, blood counts, and antiepileptic drug concentrations were measured during 133 pregnancies of 125 women with epilepsy. There was an inverse correlation between serum folate concentrations and concentrations of phenytoin and phenobarbitone. The number of epileptic seizures during pregnancy showed no association with serum folate concentrations. No cases of maternal tissue folate deficiency or fetal damage attributable to low maternal serum folate were observed. Maternal serum folate concentrations for infants with structural birth defects, "fetal hydantoin syndrome," or perinatal death were similar to those for healthy babies. A low dose (100 to 1000 micrograms daily) of folate supplement appeared sufficient for pregnant women with epilepsy despite the antifolic action of antiepileptic medication. Monitoring folate concentrations in pregnant women with high serum concentrations of phenytoin or phenobarbitone is recommended.
Howe AM, Webster WS, Lipson AH, Halliday JL, Sheffield LJ. Binder's syndrome due to prenatal vitamin K deficiency: a theory of pathogenesis. Aust Dent J 1992 Dec;37(6):453-460.
Abstract: There is evidence that vitamin K-deficiency during human pregnancy can be caused by the therapeutic use of warfarin or phenytoin. The pregnancy histories of three cases of Binder's syndrome are reported. One was associated with warfarin exposure, one with phenytoin exposure and one with alcohol abuse. It is proposed that Binder's syndrome can be caused by prenatal exposure to agents that cause vitamin K-deficiency. Sprague-Dawley rats were treated from postnatal day 1 to 12 weeks with daily doses of warfarin (100 mg/kg) and concurrent vitamin K1 (10 mg/kg). This regimen creates a net extra-hepatic vitamin K-deficiency. The treated rats developed with a distinct facial appearance characterized by a markedly reduced snout. Histological examination showed that the normally non-calcified septal cartilage was extensively calcified. It is proposed that normal growth of the septal cartilage is necessary for the development of the profile of the nose and midface and that normal growth will only take place while the septal cartilage is uncalcified.
Iivanainen M, Savolainen H. Side effects of phenobarbital and phenytoin during long-term treatment of epilepsy. Acta Neurol Scand Suppl 1983;97:49-67.
Abstract: Phenobarbital and phenytoin have good antiepileptic effect, but clinically significant untoward effects occur during their long-term use. Phenobarbital may cause hyperactivity, behavioral problems, sedation, and even dementia; these effects are dose related to some extent. Side effects of phenytoin include sedation, a cerebellar syndrome, phenytoin encephalopathy, psychosis, locomotor dysfunction, hyperkinesia, megaloblastic anemia, decreased serum folate level, decreased bone mineral content, liver disease, IgA deficiency, gingival hyperplasia, and a lupus-like hypersensitivity syndrome. Especially susceptible to the neurotoxic effects of phenytoin are epileptic children with severe brain damage who are on multiple drugs. In those children, balance disturbance may develop and be followed by gradual loss of locomotion. Among 131 mentally retarded epileptic patients, phenytoin intoxication occurred in 73 (56%), of whom 18 experienced persistent loss of locomotion. There is experimental evidence that the toxic action of phenytoin lies at the cellular level, predominantly in the cerebellum. Many experts avoid the long-term use of phenytoin because of its insidious and potentially dangerous side effects.
Jiao FY, Gao DY, Takuma Y, Wu S, Liu ZY, Zhang XK, Lieu NS, Ge ZL, Chui W, Li HR, Cao YM, Bai AN, Liu SB. Randomized, controlled trial of high-dose intravenous pyridoxine in the treatment of recurrent seizures in children. Pediatr Neurol 1997 Jul;17(1):54-57.
Abstract: To determine the efficacy of pyridoxine in treating seizures, 90 infants and children with recurrent convulsions primarily due to acute infectious diseases were enrolled in the present study. Forty patients were treated with high-dose pyridoxine (30 or 50 mg/kg/day) by intravenous infusion, and 50 subjects served as controls. Antiepileptic drugs and other therapies were similar in the two groups except for pyridoxine. Clinical efficacy criteria were based on the frequency of convulsions per day and on the duration of individual seizures after therapy was initiated. The results indicated that total response rates in the pyridoxine group and control group were 92.5% and 64%, respectively (chi-square = 14.68, P < .001). After initiation of therapy, seizures resolved after 2.4 +/- 1.4 days in the pyridoxine group and after 3.7 +/- 2.0 days in the control group (t = 3.67, P < .001). No adverse effects of pyridoxine were apparent during the observation period. We conclude that pyridoxine is an effective, safe, well-tolerated, and relatively inexpensive adjunct to routine antiepileptic drugs for treatment of recurrent seizures in children.
Keith DA, Gundberg CM, Japour A, Aronoff J, Alvarez N, Gallop PM. Vitamin K-dependent proteins and anticonvulsant medication. Clin Pharmacol Ther 1983 Oct;34(4):529-532.
Abstract: Certain anticonvulsant drugs, especially phenytoin and phenobarbital, interfere with vitamin K metabolism as indicated by a raised serum osteocalcin level. This finding may be of importance in the pathogenesis of side effects of these medications.
Keith DA, Gallop PM. Phenytoin, hemorrhage, skeletal defects and vitamin K in the newborn. Med Hypotheses 1979 Dec;5(12):1347-1351.
Abstract: The vitamin K-dependent hemostatic factors are present in reduced quantities at birth and may decrease further in the first few days of life. Administration of vitamin K1 on day 1 prevents hemorrhagic disease of the newborn. Maternal ingestion of anticonvulsants puts the newborn at greater risk from hemorrhage, possibly as a result of induction of fetal microsomal enzymes with a resultant increased oxidative degradation of vitamin K which gives rise to a vitamin K deficiency and other concomitant clinical results, for example skeletal defects. Evidence for this sequence of events is presented and the widespread effect of vitamin K deficiency on the fetus is discussed.
Lewis DP, Van Dyke DC, Stumbo PJ, Berg MJ. Drug and environmental factors associated with adverse pregnancy outcomes. Part I: Antiepileptic drugs, contraceptives, smoking, and folate. Ann Pharmacother 1998 Jul-Aug;32(7-8):802-817. (Review)
Abstract: OBJECTIVE: Part I of this review examines the relationship between antiepileptic drugs (AEDs) and pregnancy outcomes. Drug-induced folate deficiency and the role of AED metabolism are emphasized. Part II will discuss periconceptional folate supplementation for prevention of birth defects. Part III will discuss the mechanism of folate's protective effect, therapeutic recommendations, compliance, and cost. DATA SOURCES: A MEDLINE search was conducted for journal articles published through December 1997. Additional sources were obtained from Current Contents and citations from the references obtained. Search terms included phenytoin, carbamazepine, phenobarbital, primidone, valproic acid, oral contraceptives, clomiphene, drug-induced abnormalities, spina bifida, anencephaly, neural tube defect, folate, folic acid, and folic acid deficiency. STUDY SELECTION: Relevant animal and human studies examining the effects of AEDs, smoking, and oral contraceptives on folate status and pregnancy outcome are reviewed. DATA EXTRACTION: Studies and case reports were interpreted. Data extracted included dosing, serum and red blood cell folate concentrations, teratogenicity of anticonvulsant medications, metabolism of AEDs and folate, and genetic susceptibility to AED-induced teratogenicity. DATA SYNTHESIS: Low serum and red blood cell folate concentrations are associated with adverse pregnancy outcomes. Decreases in serum folate are seen with AEDs, oral contraceptives, and smoking. Since similar birth defects are observed with multiple AEDs, metabolism of aromatic AEDs to epoxide metabolites and genetic factors may play a role in teratogenesis. CONCLUSIONS: Adequate prepregnancy planning is essential for women who have epilepsy. Women receiving folate-lowering drugs may be at increased risk of adverse pregnancy outcomes. Therefore, epileptic women contemplating pregnancy should be treated with the minimum number of folate-lowering drugs possible and receive folic acid supplementation.
Lewis DP, Van Dyke DC, Stumbo PJ, Berg MJ. Drug and environmental factors associated with adverse pregnancy outcomes. Part I: Antiepileptic drugs, contraceptives, smoking, and folate. Ann Pharmacother 1998 Jul-Aug;32(7-8):802-817.
Abstract: OBJECTIVE: Part I of this review examines the relationship between antiepileptic drugs (AEDs) and pregnancy outcomes. Drug-induced folate deficiency and the role of AED metabolism are emphasized. Part II will discuss periconceptional folate supplementation for prevention of birth defects. Part III will discuss the mechanism of folate's protective effect, therapeutic recommendations, compliance, and cost. DATA SOURCES: A MEDLINE search was conducted for journal articles published through December 1997. Additional sources were obtained from Current Contents and citations from the references obtained. Search terms included phenytoin, carbamazepine, phenobarbital, primidone, valproic acid, oral contraceptives, clomiphene, drug-induced abnormalities, spina bifida, anencephaly, neural tube defect, folate, folic acid, and folic acid deficiency. STUDY SELECTION: Relevant animal and human studies examining the effects of AEDs, smoking, and oral contraceptives on folate status and pregnancy outcome are reviewed. DATA EXTRACTION: Studies and case reports were interpreted. Data extracted included dosing, serum and red blood cell folate concentrations, teratogenicity of anticonvulsant medications, metabolism of AEDs and folate, and genetic susceptibility to AED-induced teratogenicity. DATA SYNTHESIS: Low serum and red blood cell folate concentrations are associated with adverse pregnancy outcomes. Decreases in serum folate are seen with AEDs, oral contraceptives, and smoking. Since similar birth defects are observed with multiple AEDs, metabolism of aromatic AEDs to epoxide metabolites and genetic factors may play a role in teratogenesis. CONCLUSIONS: Adequate prepregnancy planning is essential for women who have epilepsy. Women receiving folate-lowering drugs may be at increased risk of adverse pregnancy outcomes. Therefore, epileptic women contemplating pregnancy should be treated with the minimum number of folate-lowering drugs possible and receive folic acid supplementation.
Lewis DP, Van Dyke DC, Willhite LA, Stumbo PJ, Berg MJ. Phenytoin-folic acid interaction. Ann Pharmacother 1995 Jul-Aug;29(7-8):726-735. (Review)
Abstract: OBJECTIVE: To review information regarding the dual and interdependent drug-nutrient interaction between phenytoin and folic acid and other literature involving phenytoin and folic acid. DATA SOURCES: Information was retrieved from a MEDLINE search of English-language literature conducted from 1983 (time of the last review) to March 1995. Search terms included folic acid, phenytoin, and folic acid deficiency. Additional references were obtained from Current Contents and from the bibliographies of the retrieved references. STUDY SELECTION: All human studies examining the effects of phenytoin on serum folate concentrations and folic acid supplementation on serum phenytoin concentrations were selected. These included studies of patients with epilepsy and healthy volunteers as well as case reports. Case reports were included because of the extensive length of time needed to study this drug interaction. DATA EXTRACTION: Data extracted included gender, dosing, serum folate concentrations if available, pharmacokinetics, and adverse events. DATA SYNTHESIS: Serum folate decreases when phenytoin therapy is initiated alone with no folate supplementation. Folic acid supplementation in folate-deficient patients with epilepsy changes the pharmacokinetics of phenytoin, usually leading to lower serum phenytoin concentrations and possible seizure breakthrough. Folate is hypothesized to be a cofactor in phenytoin metabolism and may be responsible for the "pseudo-steady-state," which is a concentration where phenytoin appears to be at steady-state, but in reality, is not. Phenytoin and folic acid therapy initiated concomitantly prevents decreased folate and phenytoin obtains steady-state concentrations sooner. CONCLUSIONS: Folic acid supplementation should be initiated each time phenytoin therapy commences because of the hypothesized cofactor mechanism, decreased adverse effects associated with folate deficiency, and better seizure control with no perturbation of phenytoin pharmacokinetics.
Livingston S. Long-term anticonvulsant therapy and vitamin D metabolism.
JAMA 1983;249:939.
McLaughlin WS, Ball DE, Seymour RA, Kamali F, White K. The pharmacokinetics of phenytoin in gingival crevicular fluid and plasma in relation to gingival overgrowth.
J Clin Periodontol 1995 Dec;22(12):942-945.
Abstract: The aim of this study was to determine whether phenytoin (PHT) could be detected in gingival crevicular fluid (GCF), and to relate its concentration to both plasma level and degree of gingival overgrowth. 23 patients medicated with phenytoin for at least 6 months were clinically examined for signs of periodontal disease and gingival overgrowth. 12 patients out of these demonstrated clinically significant overgrowth and their plaque scores and gingival inflammation were greater than for the non-overgrowth group (p < 0.001). Phenytoin concentrations were determined by high performance liquid chromatography, and was detected in GCF. There was a significant correlation between the GCF and plasma phenytoin concentrations (p < 0.05), but it was not related to the extent of gingival overgrowth. Inflammation increased the GCF volume, but was not a determinant of GCF phenytoin concentration. It is concluded that effusion of phenytoin into GCF is regulated by the plasma levels of the drug, but its concentration in GCF is not related to the incidence of gingival overgrowth.
Mimaki T, Walson PD, Haussler MR. Anticonvulsant therapy and vitamin D metabolism: evidence for different mechanisms for phenytoin and phenobarbital. Pediatr Pharmacol (New York) 1980;1(2):105-112.
Abstract: Combined therapy of epileptic children with phenobarbital (PB) and phenytoin (DPH) significantly decreased serum 25-hydroxyvitamin D (25-OH-D) levels, whereas PB alone significantly increased serum 25-OH-D levels after one to two months of therapy [Sumi et al, 1978]. Studies were conducted in rats to test the hypothesis suggested by the human studies that DPH and PB had different effects on Vitamin D metabolism. Male Wistar rats treated for five days with PB (75 mg/kg/day) had significantly (P less than 0.05) decreased 1,25-dihydroxyvitamin D (1,25-(OH)2D) levels (7.1 +/- 1.6 ng/dl, mean +/- SD) compared to controls (12.0 +/- 4.0 ng/dl) and significantly (P less than 0.005) increased conversion of [3H]-vitamin D into [3H]-25-OH-D and [3H]-24,25-(OH)2D, but no increased conversion into [3H]-25-(OH)2D. Age- and weight-matched rats treated for five days with DPH (75 mg/kg/day), however, had significantly (P less than 0.03) decreased 25-OH-D levels (41.9 +/- 5.7 ng/ml) compared to controls (52.4 +/- 4.4 ng/ml) and significantly (P less than 0.01) increased conversion into [3H]-1,25-(OH)2D. These results are consistent with clinical data, which suggest that different alterations in vitamin D metabolism occur after short-term DPH versus PB therapy.
Nulman I, Laslo D, Koren G. Treatment of epilepsy in pregnancy. Drugs 1999 Apr;57(4):535-544.
Abstract: Pregnant women with epilepsy constitute 0.5% of all pregnancies. Proper seizure control is the primary goal in treating women with epilepsy. The commonly used anticonvulsants are established human teratogens. Factors such as epilepsy, anticonvulsant-induced teratogenicity, patient's genetic predisposition and the severity of convulsive disorder may attribute to adverse pregnancy outcome for the children of women with epilepsy. Anticonvulsant interaction with folic acid and phytomenadione (vitamin K) metabolism may lead to an increased risk for neural tube defect and early neonatal bleeding. Psychological, hormonal and pharmacokinetic changes in pregnancy may escalate seizure activity. Preconceptional counselling should include patient education to ensure a clear understanding of risks of uncontrolled seizures and possible teratogenicity of anticonvulsants. Genetic counselling should be performed if both parents have epilepsy or the disease is inherited. Seizure control should be achieved at least 6 months prior to conception and, if clinically possible, by the lowest effective dose of a single anticonvulsant according to the type of epilepsy. The new anticonvulsants are not recommended in pregnancy and require further research to prove their safety in humans. Folic acid 5 mg/day should be administered 3 months before conception and during the first trimester to prevent folic acid deficiency-induced malformations. Antenatal management should include assessment of patients for anticonvulsant-associated birth defects through detailed ultrasound examination and levels of maternal serum alpha-fetoproteins. Therapeutic drug monitoring should be performed monthly, or as clinically indicated. If phenobarbital, carbamazepine or phenytoin is administered, maternal phytomenadione supplementation should begin 4 weeks before the expected date of delivery. In order to prevent convulsions during labour, proper seizure control should be achieved during the third trimester. Benzodiazepines or phenytoin are found to be effective for seizure cessation during labour and delivery. Phytomenadione should be administered immediately after birth to the newborn. The neonate should be assessed carefully for epilepsy and anticonvulsant-associated dysmorphology. Advising the patient on postpartum management regarding contraception and breast-feeding will help maximise the best possible outcome for the newborn and mother. With proper preconceptional, antenatal and postpartum management up to 95% of these pregnancies have been reported to have favourable outcomes.
Ogawa Y, Kaneko S, Otani K, Fukushima Y. Serum folic acid levels in epileptic mothers and their relationship to congenital malformations. Epilepsy Res 1991 Jan-Feb;8(1):75-78.
Abstract: Folic acid levels during pregnancy and in pre-pregnancy were determined in 51 epileptic mothers and those of matched controls. The serum folic acid (SF) levels of epileptic mothers were significantly lower than those of controls in all study periods. The SF levels of mothers of malformed offspring were significantly lower than those of mothers of normal offspring in the 1st and 2nd trimesters of pregnancy. These results suggest that SF concentrations are implicated in congenital malformations in the offspring of epileptic mothers.
Pack AR. Effects of folate mouthwash on experimental gingivitis in man. J Clin Periodontol 1986 Aug;13(7):671-676.
Patrini C, Perucca E, Reggiani C, Rindi G. Effects of phenytoin on the in vivo kinetics of thiamine and its phosphoesters in rat nervous tissues.
Brain Res 1993 Nov 19;628(1-2):179-186.
Abstract: The in vivo effects of chronic (30 days) and subchronic (10 days) intragastric treatment with phenytoin (PHT) (500 mg/kg) b.wt., suspended in 10% arabic gum water solution) on the uptake and metabolism of thiamine (T), T monophosphate (TMP) and T pyrophosphate (TPP) were evaluated in rat nervous regions (cerebral cortex, brainstem, cerebellum and sciatic nerve) by determining the radioactivity of T and its phosphoesters in plasma and tissues at fixed time intervals (0.25-240 h) after an i.p. injection of thiazole-[2-14C]thiamine (30 micrograms: 1.25 microCi). A nutritionally adequate diet containing T in excess was given to the animals in order to produce a virtually stable content of T compounds in the tissues. Analytical data were processed by using a compartmental model which allowed the calculation of fractional rate constants (FRC), turnover rates (TR) and turnover times. Compared with vehicle-treated controls, animals treated chronically with PHT exhibited lower levels of radiolabelled T compounds in all nervous regions except for the cerebral cortex. These alterations were not found in animals receiving subchronic treatment. Evaluation of FRC values indicated that PHT-induced effects on T metabolism differed depending on the length of PHT treatment and the nervous region considered. Overall, PHT appeared to interfere mainly with T and TMP uptake, TPP dephosphorylation to TMP and TPP turnover times, these effects being particularly prominent in the cerebellum and in the brainstem of chronically treated animals. Since all changes in T uptake and metabolism were observed in the absence of overt behavioural toxicity, these findings may have potential clinical relevance in highlighting possible mechanisms by which PHT therapy can alter brain metabolism.
Raju GB, Behari M, Prasad K, Ahuja GK. Randomized, double-blind, placebo-controlled, clinical trial of D-alpha-tocopherol (vitamin E) as add-on therapy in uncontrolled epilepsy.
Epilepsia 1994 Mar-Apr;35(2):368-732.
Abstract: In a double-blind, cross-over trial, vitamin E, and placebo were compared as add-on therapy in 43 patients with uncontrolled epilepsy. The study consisted of a 3-month baseline period followed by two treatment phases of vitamin E or placebo with cross-over to the second phase after 3 months. No significant side effects were noted during the study. Mean seizure frequency in the baseline period was 15.9 +/- 10.5 as compared with 11.8 +/- 10.9 during the placebo phase and 13.7 +/- 11.1 during the vitamin E phase. No significant change in seizure frequency was observed with vitamin E as compared with placebo (p > 0.1). Similar observation was noted in subgroups with generalized seizures (n = 25), partial with secondarily generalized seizures (n = 11), or complex partial seizures (CPS) (n = 7). This study did not corroborate the earlier claims of therapeutic efficacy of vitamin E as add-on therapy in refractory epilepsy in adults.
Reynolds EH, Chanarin I, Milner G, Matthews DM. Anticonvulsant therapy, folic acid and vitamin B12 metabolism and mental symptoms. Epilepsia 1966 Dec;7(4):261-270.
Rivey MP, Schottelius DD, Berg MJ. Phenytoin-folic acid: a review. Drug Intell Clin Pharm 1984 Apr;18(4):292-301. (Review)
Abstract: The nutrient-drug interaction between folate and phenytoin is a two-way interaction. Folate deficiency resulting from long-term phenytoin therapy is a common occurrence, but progression of the deficiency to a megaloblastic anemia is rare. However, there are data to suggest nonanemic folate deficiency may be detrimental to the patient. Several mechanisms have been proposed to explain the ability of phenytoin to deplete body folate. The supplementation of folic acid to folate-deficient patients taking phenytoin has been shown to result in lowered serum concentrations of phenytoin, and possibly loss of control of the seizure disorder. Folate appears to be associated with the hepatic metabolism of phenytoin, although the effect of folic acid supplementation on phenytoin elimination kinetics is suggested to be individualized.
Roe D. Drug-Induced Nutritional Deficiencies, 2nd Ed. Westport, CT: ARI Publishing, 1985, 249. (Review)
Schwaninger M, Ringleb P, Winter R, Kohl B, Fiehn W, Rieser PA, Walter-Sack I. Elevated plasma concentrations of homocysteine in antiepileptic drug treatment.
Epilepsia 1999 Mar;40(3):345-350.
Abstract: PURPOSE: Homocysteine is an experimental convulsant and an established risk factor in atherosclerosis. A nutritional deficiency of vitamin B6, vitamin B12, or folate leads to increased homocysteine plasma concentrations. During treatment with carbamazepine (CBZ), phenytoin, or phenobarbital, a deficiency in these vitamins is common. The objective of the study was to test the hypothesis that antiepileptic drug (AED) treatment is associated with increased homocysteine plasma concentrations. METHODS: A total of 51 consecutive outpatients of our epilepsy clinic receiving stable, individually adjusted AED treatment and 51 sex- and age-matched controls were enrolled in the study. Concentrations of total homocysteine and vitamin B6 were measured in plasma; vitamin B12 and folate were measured in the serum of fasted subjects. RESULTS: Patients and controls differed significantly in concentrations of folate ( 13.5+/-1.0 vs. 17.4+/-0.8 nM and vitamin B6 (39.7+/-3.4 vs. 66.2+/-7.5 nM), whereas serum concentrations of vitamin B12 were similar. The homocysteine plasma concentration was significantly increased to 14.7+/-3.0 microM in patients compared with controls (9.5+/-0.5 microM; p < 0.05, Wilcoxon rank-sum test). The number of patients with concentrations of >15 microM was significantly higher in the patient group than among controls. The same result was obtained if only patients with CBZ monotherapy were included. Patients with increased homocysteine plasma concentrations had lower folate concentrations. CONCLUSIONS: These data support the hypothesis that prolonged AED treatment may increase plasma concentrations of homocysteine, although the alternative explanation that increased homocysteine plasma concentrations are associated with the disease and not the treatment cannot be completely excluded at the moment.
Shafer RB, Nuttall FQ. Calcium and folic acid absorption in patients taking anticonvulsant drugs. J Clin Endocrinol Metab 1975 Dec;41(06):1125-1129.
Abstract: Calcium and folic acid absorption were studied in 28 adult male epileptics on chronic anticonvulsant therapy. In 16 patients on diphenylhydantoin alone, calcium absorption was abnormal in 9. In 12 patients on both diphenylhydantoin and phenobarbital, calcium absorption was abnormal in 3 patients. Folic acid (3H-PGA) absorption was normal in all but one patient, while serum folate (less than 6.4 ng/ml) was reduced in all patients. Hypocalcemia (less than 8.5 mg/100 ml) occurred in only 2 patients, while serum alkaline phosphatase was elevated in 7 patients. These findings support the proposal that rickets and osteomalacia reported in patients on chronic anticonvulsant therapy results from reduced calcium absorption. The effect of these drugs appears to be the acceleration of the metabolism of vitamin D and an increase in the excretion of polar metabolites. This may result in reduced levels of 25-hydroxycholecalciferol and 1,25-dihydroxycholecalciferol which are necessary for normal absorption of calcium. Since calcium absorption may be impaired secondary to a relative vitamin D deficiency, a supplemental increase in vitamin D intake by patients on anticonvulsant drugs is recommended.
Seymour RA, Thomason JM, Ellis JS. The pathogenesis of drug-induced gingival overgrowth. J Clin Periodontol
1996 Mar;23(3 Pt 1):165-175. (Review)
Abstract: Gingival overgrowth is a well-documented unwanted effect, associated with phenytoin, cyclosporin, and the calcium channel blockers. The pathogenesis of drug-induced gingival overgrowth is uncertain, and there appears to be no unifying hypothesis that links together the 3 commonly implicated drugs. In this review, we consider a multifactorial model which expands on the interaction between drug and/or metabolite, with the gingival fibroblasts. Factors which impact upon this model include age, genetic predisposition, pharmacokinetic variables, plaque-induced inflammatory and immunological changes and activation of growth factors. Of these, genetic factors which give rise to fibroblast heterogeneity, gingival inflammation, and pharmacokinetic variables appear to be significant in the expression of gingival overgrowth. A more thorough understanding of the pathogenesis of this unwanted effect will hopefully elucidate appropriate mechanisms for its control.
Shane-McWhorter L, Cerveny JD, MacFarlane LL, Osborn C. Enhanced metabolism of levonorgestrel during phenobarbital treatment and resultant pregnancy.
Pharmacotherapy 1998 Nov-Dec;18(6):1360-1364.
Abstract: Levonorgestrel implants (Norplant) are an alternative to oral contraceptives and medroxyprogesterone acetate intramuscular injections. An interaction may exist between levonorgestrel and agents that induce the hepatic microsomal enzyme system. A 21-year-old woman with a history of a seizure disorder, treated with phenobarbital, who received levonorgestrel implants became pregnant. After a normal delivery, she took oral contraceptives concomitantly with phenobarbital. Although she was educated about the importance of a backup method of contraception, the woman again became pregnant and delivered twins. A recent national survey of neurologists and obstetricians was conducted evaluating prescriber knowledge of interactions between oral contraceptives and anticonvulsants. Only 4% of neurologists and zero percent of obstetricians knew all the interactions between the six most commonly prescribed anticonvulsants and oral contraceptives. This case supports the importance of continued patient and prescriber education regarding the possibility of drug-drug interactions in women taking anticonvulsants and hormonal contraceptives.
Smith DB, Racusen LC. Folate metabolism and the anticonvulsant efficacy of phenobarbital. Arch Neurol 1973 Jan;28(1):18-22.
Steinberg SC, Steinberg AD. Phenytoin-induced gingival overgrowth control in severely retarded children.
J Periodontol 1982 Jul;53(7):429-433.
Abstract: Nineteen severely retarded children were studied to evaluate distribution, severity and control of phenytoin-induced gingival overgrowth (PIGO). Observations included the Plaque Index, the Gingival Index and the PIGO Index. Eighteen of these patients had gingival overgrowth with 47% having the severest type of involvement and all having severe overgrowth in the posterior regions. Elimination of topical, oral contact of phenytoin did not appear to alter gingival overgrowth. Use of SnF2 as an antiplaque agent significantly decreased plaque and overgrowth scores. Use of an electric toothbrush quite significantly decreased gingival inflammation, plaque scores and gingival overgrowth.
Schwaninger M, Ringleb P, Winter R, Kohl B, Fiehn W, Rieser PA, Walter-Sack I. Elevated plasma concentrations of homocysteine in antiepileptic drug treatment. Epilepsia 1999 Mar;40(3):345-350.
Abstract: PURPOSE: Homocysteine is an experimental convulsant and an established risk factor in atherosclerosis. A nutritional deficiency of vitamin B6, vitamin B12, or folate leads to increased homocysteine plasma concentrations. During treatment with carbamazepine (CBZ), phenytoin, or phenobarbital, a deficiency in these vitamins is common. The objective of the study was to test the hypothesis that antiepileptic drug (AED) treatment is associated with increased homocysteine plasma concentrations. METHODS: A total of 51 consecutive outpatients of our epilepsy clinic receiving stable, individually adjusted AED treatment and 51 sex- and age-matched controls were enrolled in the study. Concentrations of total homocysteine and vitamin B6 were measured in plasma; vitamin B12 and folate were measured in the serum of fasted subjects. RESULTS: Patients and controls differed significantly in concentrations of folate ( 13.5+/-1.0 vs. 17.4+/-0.8 nM and vitamin B6 (39.7+/-3.4 vs. 66.2+/-7.5 nM), whereas serum concentrations of vitamin B12 were similar. The homocysteine plasma concentration was significantly increased to 14.7+/-3.0 microM in patients compared with controls (9.5+/-0.5 microM; p < 0.05, Wilcoxon rank-sum test). The number of patients with concentrations of >15 microM was significantly higher in the patient group than among controls. The same result was obtained if only patients with CBZ monotherapy were included. Patients with increased homocysteine plasma concentrations had lower folate concentrations. CONCLUSIONS: These data support the hypothesis that prolonged AED treatment may increase plasma concentrations of homocysteine, although the alternative explanation that increased homocysteine plasma concentrations are associated with the disease and not the treatment cannot be completely excluded at the moment.
Sullivan C, Capaldi N, Mack G, Buchanan N. Seizures and natural vitamin E.
Med J Austr 1990 Jun 4;152(11):613-614. (Letter)
Tjellesen L, Hummer L, Christiansen C, Rodbro P. Different metabolism of vitamin D2/D3 in epileptic patients treated with phenobarbitone/phenytoin. Bone 1986;7(5):337-342.
Abstract: Serum concentrations of vitamin D metabolites were measured before and during treatment with either vitamin D2 or vitamin D3, 4000 IU per day for 24 weeks, in 22 epileptic outpatients receiving phenobarbitone/phenytoin. The serum concentration of total 1,25(OH)2D did not change during the treatment period in any of the treatment groups. On the other hand, in the vitamin D2 group, serum 25(OH)D2, total 25(OH)D, and 24,25(OH)2D increased significantly during the trial, whereas serum concentrations of the vitamin D3 metabolites were unchanged. In the vitamin D3 group, serum concentrations of the vitamin D3 metabolites increased significantly, whereas the vitamin D3 metabolite levels remained unchanged. However, vitamin D3 treatment resulted in a 2-4-fold greater increase in serum concentrations compared to vitamin D2 treatment. Treatment with vitamin D2 and vitamin D3 in the same dose in IU results in considerably different serum concentrations of the vitamin D metabolites.
Wiehl W, Hart LL. Vitamin E as an anticonvulsant. DICP 1991 Apr;25(4):362-363. (Review)
Williams C, Netzloff M, Folkerts L, Vargas A, Garnica A, Frias J. Vitamin D metabolism and anticonvulsant therapy: effect of sunshine on incidence of osteomalacia.
Southern Med J 1984 Jul;77(7):834-836, 842.
Abstract: To study the association between anticonvulsant therapy and osteomalacia or rickets, we evaluated 450 epileptic patients receiving anticonvulsants and residing in an institution for the mentally retarded in Florida. Fifty-five of them with increased mean serum alkaline phosphatase and mildly depressed mean serum calcium levels were identified as being at risk for having osteomalacia. None of them, however, had low serum levels of vitamin D (25[OH]D) or radiologic evidence of osteomalacia or rickets. In contrast to reports from northern climates, we found minimal evidence of anticonvulsant-induced bone disease. The study suggests that exposure to sunshine in our patients probably prevented the development of anticonvulsant-induced osteomalacia or rickets.
Yerby MS. Problems and management of the pregnant woman with epilepsy. Epilepsia 1987;28 Suppl 3:S29-36. (Review)
Abstract: Pregnancies occurring in women who are epileptic are considered to be high risk. These women are at increased risk of seizures during pregnancy, labor, and delivery and of pregnancy complications and adverse pregnancy outcomes. Pregnancy alters the pharmacokinetics of anticonvulsant drugs, the levels of which decline as pregnancy advances. Not all drugs are altered in a similar manner, however. The rate of congenital malformations in infants of epileptic mothers is 2.4 times higher than in the general population. Malformations occur with all of the commonly used anticonvulsant drugs. The possible mechanisms of teratogenicity include folic acid antagonism, fetal tissue binding, and toxic effects of metabolic intermediates. Therapy with more than one drug increases the risk of congenital malformations. A unique hemorrhagic phenomenon in the infants of epileptic mothers has been reported and appears to be the result of a deficiency of vitamin K-dependent clotting factors. When taken by a pregnant woman, all antiepileptic drugs except valproic acid manifest themselves in breast milk, but only if the infant exhibits evidence of sedation should breastfeeding be discontinued. The dilemma for the physician treating the pregnant epileptic woman is to protect the mother from seizures and the fetus from unnecessary exposure to anticonvulsant medications.

