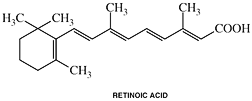

Retinoic Acid
Brand Names: Accutane, Roaccutane
Clinical Names: Isotretinoin, Retinoic Acid
Summary
generic name: Retinoic acid (naturally occurring)
synonym: Isotretinoin (synthetic)
trade name: Accutane®, Roaccutane®
type of drug: Retinoic acid is a modified form of the naturally-occurring vitamin A molecule.
used to treat: Severe acne.
general caution: As with other retinoids, reliable contraception is mandatory for women of childbearing age.
(Chen C, et al. J Clin Pharmacol 1996 Sep;36(9):799-808; Meigel WN. Dermatology 1997;195 Suppl 1:22-28; Mitchell AA.
Drug Saf. 1992 Mar-Apr;7(2):79-85.)
overview of interactions:
• nutrient affecting drug toxicity: Vitamin A
• nutrient affected by drug: Calcium
Interactions
nutrient affecting drug toxicity: Vitamin A
• mechanism: Vitamin A supplementation increases levels of retinoic acid compounds in human plasma.
(Wiegand UW, et al. Int J Vitam Nutr Res 1998;68(6):411-416; Chen C, et al. J Clin Pharmacol
1996 Sep;36(9):799-808; Eckhoff C, Nau H. Arch Toxicol. 1990;64(6):502-503.)
• nutritional concerns: Conservative practice would suggest that supplemental use of vitamin A be avoided while taking Accutane. Even though no studies have confirmed interactions, the structural similarities between and significant toxicities of both Accutane and natural vitamin A warrant caution. Consequently, supplementation of vitamin A by anyone using Accutane should only be undertaken under the supervision of an appropriately trained healthcare professional. These concerns are amplified for women of child-bearing age as the risk of birth defects inherent to either substance is increased by their simultaneous use.
nutrient affected by drug: Calcium
• research: Long-term or high-dose administration of vitamin A derivatives (retinoids) may produce a variety of skeletal side-effects in humans. Kindmark et al investigated the early effects of oral isotretinoin therapy on bone turnover and calcium homeostasis in eleven consecutive patients with nodulocystic acne. They found that markers of bone turnover and urine levels of calcium and hydroxyproline decreased significantly within five days of treatment. There was also a statistically significant decrease in serum calcium, with a minimum on day five, and a marked increase in serum parathyroid hormone. However, with continued treatment the abnormal levels of these markers returned to baseline values within 14 days.
(Kindmark A, et al. Acta Derm Venereol 1998 Jul;78(4):266-269.)
• nutritional concerns: Individuals taking retinoic acid should consult their prescribing physician and/or a nutritionally trained healthcare professional about the possible benefits of taking supplemental calcium during the course of therapy. A daily dosage of 1000 mg is within the common range of supplementation and is generally considered safe.
Please read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
Chen C, Mistry G, Jensen B, Heizmann P, Timm U, van Brummelen P, Rakhit AK.Pharmacokinetics of retinoids in women after meal consumption or vitamin A supplementation.
J Clin Pharmacol 1996 Sep;36(9):799-808.
Abstract: These studies were conducted to evaluate the pharmacokinetics of several retinoids after meal consumption or vitamin A supplementation to establish a reference for future assessment of teratogenic risks of retinoid therapeutic agents. In the first study, 36 healthy young female volunteers consumed single meals containing vitamin A amounts ranging from 1,305 to 169,474 IU. In the second study, 24 other female volunteers took vitamin A supplements at a dose level of 5,000, 10,000, or 25,000 IU/day for 60 days. Plasma concentrations of tretinoin, isotretinoin, 4-oxo-tretinoin, and 4-oxo-isotretinoin in samples collected during the studies were analyzed using a high-performance liquid chromatography method with ultraviolet detection. Pharmacokinetic parameters for the retinoids were calculated using model-independent methods. Plasma concentrations of tretinoin were not altered by meal consumption or vitamin A supplementation. Plasma levels of 4-oxo-tretinoin were below the assay detection limit (0.3 ng/mL) in the majority of samples collected throughout the studies. Linear relationships between dose and maximum concentration (Cmax) and dose and area under the concentration-time curve (AUC) for isotretinoin and 4-oxo-isotretinoin were derived from data from the meal study. For the most bioavailable formulation used in the supplement study, daily ingestion of 5,000 IU of vitamin A caused increases of 141 +/- 53% and 171 +/- 77% from baseline in the 24-hour AUCs of isotretinoin and 4-oxo-isotretinoin, respectively. Dose-related increases in systemic exposure to retinoids were observed after ingestion of vitamin A by means of a meal or a supplement. Findings from these studies can be used as a basis for future safety evaluations of retinoid compounds.
Eckhoff C, Nau H. Vitamin A supplementation increases levels of retinoic acid compounds in human plasma: possible implications for
teratogenesis. Arch Toxicol. 1990;64(6):502-503.
Abstract: The concentrations of retinoic acid compounds were monitored by a newly developed highly sensitive HPLC procedure in plasma of six volunteers who received 833 IU vitamin A per kg body weight per day during a 20-day period. There was a significant increase of all-trans-retinoic acid (two-fold), 13-cis-retinoic acid (7-fold) and 13-cis-4-oxoretinoic acid (5-fold) over endogenous plasma levels of these retinoids. The same compounds had previously been found after treatment with the teratogenic drug isotretinoin (Roaccutan, Accutane). Our results raise the possibility that high vitamin A intake may carry a teratogenic risk attributable to increased levels of retinoic acid compounds generated from retinol by metabolic processes.
Kindmark A, Rollman O, Mallmin H, Petren-Mallmin M, Ljunghall S, Melhus H
Oral isotretinoin therapy in severe acne induces transient suppression of biochemical markers of bone turnover and calcium homeostasis.
Acta Derm Venereol 1998 Jul;78(4):266-269.
Abstract: Although dietary vitamin A is required for normal growth and development, long-term or high-dose administration of vitamin A derivatives (retinoids) may produce a variety of skeletal side-effects in man. In this study we investigated the early effects of oral isotretinoin therapy on bone turnover and calcium homeostasis in eleven consecutive patients with nodulocystic acne. The effects on bone metabolism were correlated to radiological and bone mineral density measurements following drug therapy for six months. Markers of bone turnover, i.e. serum osteocalcin, the carboxyterminal propeptide of type I collagen, bone specific alkaline phosphatase, the carboxyterminal telopeptide of type I collagen, and urine levels of calcium and hydroxyproline decreased significantly within five days of treatment (p < 0.05). There was also a statistically significant decrease in serum calcium, with a minimum on day five, and a marked increase in serum parathyroid hormone (p < 0.05). With continued treatment, however, the abnormal levels of these markers returned to baseline values within 14 days. No significant roentgenological changes or effects on bone mineral density were found in response to the drug. The observed inhibitory effects of isotretinoin on bone turnover, despite elevated parathyroid hormone levels, indicates that the drug exerts a direct effect on bone tissue.
Meigel WN. How safe is oral isotretinoin? Dermatology 1997;195 Suppl 1:22-28; discussion 38-40. (Review)
Abstract: Since oral isotretinoin (Roaccutane/Accutane) is the only therapy to address all major acne causes, it remains the most effective antiacne therapy available. Due to this unique efficacy and its potential side effects that are predictable and can be managed easily and effectively, it is widely used also in acne patients suffering from serious systemic diseases. As the primary mechanism of action of oral isotretinoin is suppression of sebaceous gland activity, mucocutaneous side effects such as dry lips, nasal passages and eyes are predictable. Pretreatment counseling and concomitant use of moisturizing agents usually manage these side effects effectively; in unusual cases of particularly poor tolerability, dose adjustments suffice. Severe side effects are rare, the most common being aches and pains requiring no therapy, aspirin or paracetamol. As with other retinoids, reliable contraception is mandatory for women of childbearing potential. Acne patients with serious concomitant systemic disease, such as insulin-dependent diabetes, epilepsy or spina bifida, transplant patients, patients with renal failure, multiple sclerosis motor neuron disease and other can also safe be treated with a standard cumulative dose of 120 mg/kg per treatment course.
Mitchell AA. Oral retinoids. What should the prescriber known about their teratogenic hazards among women of child-bearing potential?
Drug Saf. 1992 Mar-Apr;7(2):79-85. (Review)
Wiegand UW, Hartmann S, Hummler H. Safety of vitamin A: recent results. Int J Vitam Nutr Res 1998;68(6):411-416. (Review)
Abstract: A still unresolved public health concern is that excessive vitamin A intake, like vitamin A deficiency, possibly causes birth defects not only in animals but also in man. Due to the low incidence of possibly vitamin A-related malformations in man, available data cannot convincingly define the upper safe limit of periconceptional vitamin A intake. Direct human intervention studies are not feasible for ethical reasons. Therefore, a novel approach in addressing this issue was chosen by combining teratogenicity data from a validated animal model with data on systemic exposure to vitamin A and its major metabolites in female volunteers. In a study in pregnant women endogenous plasma concentrations of vitamin A metabolites during early pregnancy ranged from 0.26 to 7.72 ng/ml. Since they did not cause any foetal malformations, retinoid plasma levels in this range can be considered non-teratogenic. Results of a trial in non-pregnant women document that daily oral vitamin A supplements of 4000, 10,000 and 30,000 IU given for 3 weeks were in the range or slightly above the range of endogenous plasma levels seen in early pregnancy. Even after a 3-week treatment with 30,000 IU/day, peak plasma levels of retinoic acid and isotretinoin were within or just slightly above the range of their physiological levels. In cynomolgus monkeys (average weight: 3-4 kg), a NOAEL (no observed adverse effect level) of 7500 IU per kg body weight and a LOAEL (lowest observed adverse effect level) for developmental toxicity of 20,000 IU/kg was found. Considering these results in the cynomolgus monkey, a dose of 30,000 IU/day should also be considered as non-teratogenic in man.