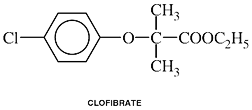

Clofibrate
Brand Names: Atromid-S
Please read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
Knodel LC, Talbert RL. Adverse effects of hypolipidaemic drugs. Med Toxicol 1987 Jan;2(1):10-32.
Abstract: Cholestyramine, colestipol, clofibrate, gemfibrozil, nicotinic acid (niacin), probucol, neomycin, and dextrothyroxine are the most commonly used drugs in the treatment of hyperlipoproteinaemic disorders. While adverse reaction data are available for all of them, definitive data regarding the frequency and severity of potential adverse effects from well-controlled trials using large numbers of patients (greater than 1000) are available only for cholestyramine, clofibrate, nicotinic acid and dextrothyroxine. In adult patients treated with cholestyramine, gastrointestinal complaints, especially constipation, abdominal pain and unpalatability are most frequently observed. Continued administration along with dietary manipulation (e.g. addition of dietary fibre) and/or stool softeners results in diminished complaints during long term therapy. Large doses of cholestyramine (greater than 32 g/day) may be associated with malabsorption of fat-soluble vitamins. Most significantly, osteomalacia and, on rare occasions, haemorrhagic diathesis are reported with cholestyramine impairment of vitamin D and vitamin K absorption, respectively. Paediatric patients have been reported to experience hyperchloraemic metabolic acidosis or gastrointestinal obstruction. Concurrent administration of acidic drugs may result in their reduced bioavailability. Serious adverse reactions to clofibrate will probably limit its role in the future. Of particular concern are ventricular arrhythmias, induction of cholelithiasis and cholecystitis, and the potential for promoting gastrointestinal malignancy which far outweigh the reported benefits in preventing new or recurrent myocardial infarction, cardiovascular death and overall death. Patients with renal disease are particularly prone to myositis, secondary to alterations in protein binding and impaired renal excretion of clofibrate. Drug interactions with coumarin anticoagulants and sulphonylurea compounds may produce bleeding episodes and hypoglycaemia, respectively. Nicotinic acid produces frequent adverse effects, but they are usually not serious, tend to decrease with time, and can be managed easily. Dermal and gastrointestinal reactions are most common. Truncal and facial flushing are reported in 90 to 100% of treated patients in large clinical trials. Significant elevations of liver enzymes, serum glucose, and serum uric acid are occasionally seen with nicotinic acid therapy. Liver enzyme elevations are more common in patients given large dosage increases over short periods of time, and in patients treated with sustained release formulations.
Powanda MC, Blackburn BS, Bostian KA, Fowler JP, Hauer EC, Pekarek RS. Clofibrate-induced alterations in zinc, iron and copper metabolism.
Biochem Pharmacol 1978 Jan 1;27(1):125-127.
Powanda MC, Henriksen EL, Ayala E, Canonico PG. Clofibrate-induced alterations in serum protein patterns.
Biochem Pharmacol. 1976 Apr 1;25(7):785-788.
Robinson C, Weigly E. Basic Nutrition and Diet Therapy. New York: Macmillan, 1984, 46-54.
Roe DA. Essential hyperlipemia with xanthomatosis: effects of cholestyramine and
clofibrate. Arch Dermatol 1968 Apr;97(4):436-445.
Vitamin E Fact Book. LaGrange, IL: VERIS (Vitamin E Research and Information Service), 1994