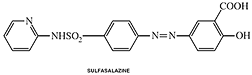
Sulfasalazine
Brand Names: Azulfidine, Kenral, Salicylazosulfapyridine.
Clinical Names: Sulfasalazine
Summary
generic name: Sulfasalazine
trade names: Azulfidine®, Kenral®, Salicylazosulfapyridine®
type of drug: Sulfonamide
used to treat: Crohn’s disease, rheumatoid arthritis or juvenile arthritis, and ulcerative colitis.
overview of interactions:
• nutrient affected by drug: Folic Acid
(Folate)
• interaction: Iron
• diet affecting drug performance: Food
• nutrient affecting drug performance: PABA
(Para-amino Benzoic Acid)
• potential interaction: Digitalis
(Foxglove) and other plants containing cardiac glycosides.
Interactions
nutrient affected by drug: Folic Acid
(Folate)
• mechanism: Sulfasalazine is well known for its role in inhibiting the absorption of dietary folicin, most likely acting as a competitive inhibitor of folate absorption. There has been some controversy as to whether folic acid by itself can cause clinically significant deficiencies of folic acid. Sulfasalazine also inhibits folate-dependent enzymes. The mechanisms by which sulphasalazine antagonizes folate metabolism are dose-dependent and, consequently, higher doses might precipitate folate deficiency. However, a strong proportion of the literature indicates a multifactorial causality for folate depletion in the affected populations with the side effects of sulfasalazine being an important but not necessarily adequate stress. The conditions for which the drug is usually prescribed are often associated with malabsorption, poor diet and chronic inflammation. For example, patients with ulcerative colitis commonly have decreased folate levels. Furthermore, any sulfasalazine-induced folic acid deficiency could contribute to higher levels of homocysteine and increased risk of cardiovascular disease.
(Baum CL, et al. J Lab Clin Med 1981 Jun;97(6):779-784; Selhub J, et al.
J Clin Invest 1978 Jan;61(1):221-224;4(3):114; Pironi L, et al. Minerva Dietol Gastroenterol
1987 Oct-Dec;33(4):307-313; Halsted CH, et al. N Engl J Med 1981;305:1513-1517; Swinson CM, et al.
Gut 1981;22:456-61; Longstreth GF, Green R. N Engl J Med 1982;306:1488; Nutr Rev. 1988 Sep;46(9):320-323; Baggott JE, et al.
Clin Exp Rheumatol 1993 Mar-Apr;11 Suppl 8:S101-105; Das KM, Dubin R. Clinical Pharmacokinetics 1976 Nov-Dec;1(6):406-425; Grindulis KA, McConkey B.
Scand J Rheumatol 1985;14(3):265-270; Haagsma CJ, et al. Ann Rheum Dis 1999 Feb;58(2):79-84; Krogh Jensen M, et al.
Scand J Clin Lab Invest 1996 Aug;56(5):421-429; Elsborg L.Dan Med Bull 1982; 29:362-365.)
• research: Although maintenance sulfasalazine use may not commonly cause clinically significant folate deficiency states, sulphasalazine inherently impairs folate absorption and subclinical tissue depletion usually occurs as a dose-related effect. Folic acid is an especially important nutrient for those individuals who typically use sulfasalazine because they usually suffer from conditions for which folic acid can have therapeutic value. Folate is well known for its role in reducing the risks for some forms of cancer. A significant aspect of this benefit may be attributable to its key role in the healthy replication of cells.
In particular, folate supplementation has been associated with a reduced risk of colon cancer in patients with ulcerative colitis while folate deficiency has been associated with an increased risk for colon cancer. For example, in a study of patients with chronic ulcerative colitis Lashner et al found a 62% lower risk of colon cancer with folate supplementation, compared to ulcerative colitis patients who did not supplement with folate . In a later study Lasher and a different team found that individuals who have ulcerative colitis and who supplement folic acid had a 55% lower risk of developing colon cancer. Although these findings were not statistically significant, they concluded that daily folate supplementation may protect against the development of neoplasia in ulcerative colitis. While many factors contribute to the occurrence of colon cancer many researchers have suggested that folic acid deficiency may increase susceptibility and that supplementation may have a preventive effect.
(Longstreth GF, Green R. Arch Intern Med 1983 May;143(5):902-904; Lashner BA, et al.
Gastroenterology 1989 Aug;97(2):255-259; Gastroenterol 1988; 94(5 part 2): A252; Ma J, et al.
Cancer Res 1997 Mar 15;57(6):1098-1102; Mason JB. J Nutr Biochem 1994;5:170-175; Cravo ML, et al. Am J
Gastroenterol. 1998 Nov;93(11):2060-2064; Mouzas IA, et al. Ital J Gastroenterol
Hepatol. 1998 Aug;30(4):421-425.)
• nutritional support: Most researchers have concluded that folate supplementation during sulfasalazine administration is recommended, especially to prevent the complication of dysplasia or cancer in ulcerative colitis. supplementation with folic acid would be appropriate for individuals taking sulfasalazine. Folate has no known risks at the suggested levels and can potentially provide a number of benefits to those taking sulfasalazine or even those simply suffering from, or predisposed to, some of the conditions it is most commonly prescribed for. Many commonly available multivitamin or B-complex formulations provide folic acid at daily doses of approximately 800 mcg. Individuals using sulfasalazine might benefit from higher doses, in the range of 1000-1200 mcg per day but should consult with their prescribing physician, pharmacist, and/or nutritionally trained healthcare professional before adopting such a regimen.
(Lashner BA, et al. Gastroenterology 1989 Aug;97(2):255-259; Ma J, et al.
Cancer Res 1997;57:1098-102; Lashner BA, et al. Gastroenterol 1997;112:29-32; Elsborg L.Dan Med Bull
1982; 29:362-365.)
interaction: Iron
• mechanism: Iron and sulfasalazine have a tendency to bind to each other in the digestive tract. As a result, the simultaneous ingestion of both substances can lead to decreased sulfasalazine absorption, decreased iron absorption, or both.
(Dukes GE Jr, Duncan BS. 1995, 24-27.)
• nutritional support: Since sulfasalazine may reduce the absorption of iron from dietary sources supplementation may be appropriate. However, individuals taking sulfasalazine should not begin supplementation with iron before consulting with their prescribing physician, pharmacist, and/or nutritionally trained healthcare professional. In any event, if iron supplementation is undertaken, no iron-containing products should be taken two hours before or after sulfasalazine. so as to minimize the potential interaction.
diet affecting drug performance: Food
• nutritional concerns: Sulfasalazine is usually prescribed to be taken after meals for best effect. Further, physicians and pharmacists generally advise patients to swallow the tablets whole in order to avoid inactivation by stomach acid.
(Threlkeld DS, ed. Sep 1997.)
nutrient affecting drug performance: PABA
(Para-amino Benzoic Acid)
• interaction: PABA-containing compounds, as well as local anesthetics derived from PABA such as procaine, may directly inhibit the activity of sulfonamides, the class of drugs of which sulfasalazine is a member.
(Drug Evaluation Subscription. Vol II, Section 13, Chapter 7, Spring 1993.)
potential interaction: Digitalis
(Foxglove) and other plants containing cardiac glycosides.
• mechanism: In looking at drug-drug interactions researchers have found that sulfasalazine may cause reduced absorption of digoxin. While this research has focussed on pharmaceutical digoxin the findings could reasonably be extrapolated to the use of herbs containing digoxin and other cardiac glycosides.
(Juhl RP, et al. Clin Pharmacol Ther. 1976 Oct;20(4):387-394.)
• herbal concerns: In the unlikely event that an individual has been prescribed cardioactive herbs by a qualified medical herbalist or a physician trained in herbal prescribing the concurrent use of sulfasalazine might create results other than expected due to a potential interaction. The use of herbs based on the action of their cardiac glycosides is a potentially dangerous practice to be undertaken only under close observation by a highly trained and experienced herbal prescriber. Naturally occurring cardiac glycosides have a limited distribution confined to a few dozen species scattered across several genera, principally the
Asclepiadaceae and Apocyanaceae. Concentrations of glycosides are generally well below 1%. Three genera contain sufficient concentrations of glycosides for commercial extraction:
Digitalis spp.- Foxgloves - (Scrophulariaceae), Urginia spp - Squills - (Liliaceae) and
Strophanthus (Apocynaceae). These plants are described as cardioactive by herbalists, as opposed to the milder cardiotonic herbs such as Hawthorn. Due to their toxicity, these herbs are not available to the public. Neither
Digitalis or Strophanthus are commonly used in herbal therapeutics and in many countries their use is legally restricted.
Convallaria majalis and Urginia maritima are listed in the British Herbal Pharmacopoeia. Their cardenolides have low cumulative toxicity compared to
Digitalis, and these plants are used by professional herbalists. For an excellent discussion of use of cardiac glycoside herbs see Weiss (1988).
Common herbs containing cardiac glycosides:
• Asclepias tuberosa (Pleurisy Root )*
• Convallaria majalis (Lily of the Valley)
• Scrophularia nodosa (Figwort) *
• Urginea maritima (Squill bulb)
Note: * These herbs contain therapeutically insignificant quantities of glycosides.
Restricted or unusual herbs containing cardiac glycosides:
• Adonis vernalis (Pheasant’s Eye)
• Apocynum cannabinum (Canadian Hemp Root) toxic
• Digitalis spp. (Foxglove) toxic
• Helleborus niger (Black Hellebore) toxic
• Helleborus viride (Christmas Rose) toxic
• Nerium oleander (Rose Laurel) toxic
• Strophanthus spp. (Ouabain, Kombe) toxic
• Thevetia neriifolia (Yellow Oleander) toxic


Please read the disclaimer concerning the intent
and limitations of the information provided here.
Do not rely solely on the information in this article.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
References
[No authors listed] Drug Evaluation Subscription. Chicago, American Medical Association, Vol II, Section 13, Chapter 7, Spring 1993.
[No authors listed] Sulfasalazine inhibits folate absorption. Nutr Rev. 1988 Sep;46(9):320-323.
Baggott JE, Morgan SL, Ha TS, Alarcon GS, Koopman WJ, Krumdieck CL. Antifolates in rheumatoid arthritis: a hypothetical mechanism of action.
Clin Exp Rheumatol 1993 Mar-Apr;11 Suppl 8:S101-105. (Review)
Baum CL, Selhub J, Rosenberg IH. Antifolate actions of sulfasalazine on intact lymphocytes.
J Lab Clin Med 1981 Jun;97(6):779-784.
Abstract: SASP, the drug most widely used for the treatment of Crohn's disease and ulcerative colitis, is a competitive inhibitor of intestinal folate metabolism and transport. Some of the therapeutic effects of the drug could be related to antifolate actions on lymphocytes, which predominate in the inflammatory reaction in inflammatory bowel diseases. Experiments were designed to examine the effect of SASP on folate-dependent systems in cultured lymphocytes. In rat spleen lymphocytes, THF-dependent conversion of glycine to serine was inhibited by SASP, with 50% inhibition occurring at 0.1 mM. Further evidence of folate antagonism was obtained with the dU suppression test, which depends on the function of a folate-dependent pathway in the de novo synthesis of DNA. Folate antagonists like methotrexate or folate depletion reduces the incorporation of dU into DNA and thus favors incorporation of [3H]thymidine into DNA by an alternate pathway. SASP inhibited the folate-dependent pathway in proliferating virally transformed human lymphocytes (Raji cells). To confirm that SASP acts as a folate antagonist in this system, THF was demonstrated to partly reverse the action of SASP. The significance of this antifolate action by SASP in intact lymphocytes deserves further study in relation to the actions of SASP in patients with inflammatory bowel disease.
Brinker F. Herb Contraindications and Drug Interactions. Sandy, OR: Eclectic Institute, 1997.
Buist RA. Drug-nutrient interactions - an overview. Intl Clin Nutr Rev 1984;4(3):114.
Cravo ML, et al. Microsatellite instability in non-neoplastic mucosa of patients with ulcerative colitis: effect of folate supplementation.
Am J Gastroenterol. 1998 Nov;93(11):2060-2064.
Das KM, Dubin R. Clinical pharmacokinetics of sulphasalazine. Clinical Pharmacokinetics 1976 Nov-Dec;1(6):406-425. (Review)
Abstract: Sulphasalazine consists of 5-aminosalicylic acid and sulphapyridine both linked together by an azo bond. Sulphasalazine is clearly useful in long-term management of ulcerative colitis and may be useful in Crohn's disease. The absorption, metabolism and excretion of sulphasalazine is similar in volunteers and patients with ulcerative colitis or Crohn's disease. Sulphasalazine serves as a vehicle to deliver its possible active components, 5-aminosalicylic acid and sulphapyridine, to the colon in higher concentrations than could be achieved by oral administration of either one alone. Sulphasalazine reaches the colon mostly unchanged and is split by gut bacteria at the azo linkage, releasing 5-aminosalicylic acid and sulphapyridine. 5-Aminosalicylic acid may act locally and is not absorbed to any great extent. On the contrary, sulphapyridine is mostly absorpbed from the colon and may act both locally, during mucosal absorption, and systemically. A positive correlation exists between serum total sulphapyridine concentration and both therapeutic efficacy and toxicity. Sulphapyridine metabolism is largely determined by inherited acetylator phenotype, either slow or fast. Slow acetylators have higher levels of free sulphapyridine and lower levels of acetylated sulphapyridine than fast acetylators, and are likely to have more toxic symptoms on equivalent doses of sulphasalazine. Therapeutic effects of sulphasalazine in ulcerative colitis and Crohn's disease correlate with serum concentrations of total sulphapyridine (20 to 50 microng/ml), and toxicity with total sulphapyridine concentration greater than 50 microng/ml. Side-effects are mostly observed among slow acetylators. In long-term therapy of ulcerative colitis doses of 2 to 3g/day of sulphasalazine are most likely to sustain remissions and avoid toxicity. During therapy with sulphasalazine, determination of acetylator phenotype and total sulphapyridine concentration can guide effective dosage and avoid side-effects. A single serum sample for free and acetylated sulphapyridine concentrations is sufficient for this purpose.
Dukes GE Jr, Duncan BS. Inflammatory bowel disease. In: Applied Therapeutics: The Clinical Use of Drugs, 6th ed. Vancouver, WA: Applied Therapeutics, 1995, 24-27.
Elsborg L. Vitamin B12 and Folic Acid in Crohn's Disease. Dan Med Bull 1982; 29:362-365.
Gomez G. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis.
Gastroenterology. 1991 Jun;100(6):1789-1790.
Grindulis KA, McConkey B. Does sulphasalazine cause folate deficiency in rheumatoid arthritis?
Scand J Rheumatol 1985;14(3):265-270.
Abstract: Sulphasalazine impairs folic acid absorption and metabolism but rarely leads to folate deficiency in inflammatory bowel disease (IBD). In rheumatoid arthritis (RA), however, serum and red cell folate concentrations are often low and sulphasalazine might stress folate metabolism. In a prospective study, 2 g sulphasalazine was compared with 500 mg penicillamine daily in 30 patients over 24 weeks. Pre-treatment serum and red cell folate concentrations were low-normal. Improvements in disease activity and haemoglobin occurred in both treatment groups, but MCV increased only in patients taking sulphasalazine. Serum and red cell folate concentrations did not change in either group. Increased MCV with sulphasalazine might therefore reflect reticulocytosis secondary to drug-induced haemolysis. The mechanisms by which sulphasalazine antagonizes folate metabolism are dose-dependent and, consequently, higher doses might precipitate folate deficiency.
Haagsma CJ, Blom HJ, van Riel PL, van't Hof MA, Giesendorf BA, van Oppenraaij-Emmerzaal D, van de Putte LB. Influence of sulphasalazine, methotrexate, and the combination of both on plasma homocysteine concentrations in patients with rheumatoid arthritis.
Ann Rheum Dis 1999 Feb;58(2):79-84.
Abstract: OBJECTIVE: To study the influence of sulphasalazine (SSZ), methotrexate (MTX), and the combination (COMBI) of both on plasma homocysteine and to study the relation between plasma homocysteine and their clinical effects. METHODS: 105 patients with early rheumatoid arthritis (RA) were randomised between SSZ (2-3 g/day), MTX (7.5-15 mg/week), and the COMBI (same dose range) and evaluated double blindly during 52 weeks. Plasma homocysteine, serum folate concentrations, and vitamin B12 were measured. The influence of the C677T mutation of the enzyme methyl-enetetrahydrofolatereductase (MTHFR) gene was analysed. RESULTS: A slight trend towards increased efficacy and an increased occurrence of minor gastrointestinal toxicity was present in the COMBI group, no differences existed clinically between SSZ and MTX. Only a slight and temporary increase in plasma homocysteine was found in the SSZ group, in contrast with the persistent rise in the MTX group and the even greater increase in the COMBI patients. Patients homozygous for the mutation in the MTHFR gene had significantly higher baseline homocysteine, heterozygous MTHFR genotype induced a significantly higher plasma homoeysteine at week 52 compared with no mutation. No correlation was found between clinical efficacy variables and homocysteine. Patients with gastrointestinal toxicity had a significantly greater increase in homocysteine. CONCLUSION: A persistent increase in plasma homocysteine concentrations was observed in patients treated with MTX alone and more pronounced in combination with SSZ, in contrast with SSZ alone. An increase in plasma homocysteine is related to the C677T mutation in MTHFR. A relation in the change in homocysteine concentrations with (gastrointestinal) toxicity was found, no relation with clinical efficacy existed.
Halsted CH, Gandhi G, Tamura T. Sulfasalazine inhibits the absorption of folates in ulcerative colitis.
N Engl J Med 1981 Dec 17;305(25):1513-1517.
Heimburger DC, Alexander CB, Birch R, Butterworth CE Jr, Bailey WC, Krumdieck CL. Improvement in bronchial squamous metaplasia in smokers treated with folate and vitamin B12. Report of a preliminary randomized, double-blind intervention trial.
JAMA 1988 Mar 11;259(10):1525-1530.
Abstract: To test whether changes in folate and vitamin B12 nutrition modify the severity of potentially premalignant lesions identified by cytology in sputum samples of smokers, we conducted a randomized, controlled prospective intervention trial in smokers with bronchial squamous metaplasia. Seventy-three men with a history of 20 or more pack-years of cigarette smoking who had metaplasia on one or more sputum samples were stratified according to smoking level and randomly assigned to four months' treatment with either placebo or 10 mg of folate plus 500 micrograms of hydroxocobalamin. Direct cytological comparison of the two groups after four months showed significantly greater reduction of atypia in the supplemented group. This provides preliminary evidence that atypical bronchial squamous metaplasia may be reduced by supplementation with folate and vitamin B12. However, the significance of these findings is tempered by substantial spontaneous variation in sputum cytologies, the small study population, the short duration of the trial, and the supraphysiological doses of folate and B12 used. The results should not be construed as pointing to a potential way of preventing lung cancer in individuals who continue to smoke or as supporting self-medication with large doses of folate or B12 by smokers.
Juhl RP, et al. Effect of sulfasalazine on digoxin bioavailability.
Clin Pharmacol Ther. 1976 Oct;20(4):387-394.
Krogh Jensen M, Ekelund S, Svendsen L. Folate and homocysteine status and haemolysis in patients treated with sulphasalazine for arthritis.
Scand J Clin Lab Invest 1996 Aug;56(5):421-429.
Abstract: In an attempt to estimate the frequency of folate deficiency and haemolysis in a group of 25 outpatients with arthritis treated with sulphasalazine (SASP), haematological measurements, including plasma total homocysteine (tHcy) which is a sensitive marker of folate deficiency, serum folate (S-folate), erythrocyte (RBC) folate, S-cobalamin and routine indices of haemolysis were performed. No patient had been taking folate-containing vitamins for at least 8 weeks prior to the study. Compared to a group of 72 healthy hospital staff, the median plasma tHcy was significantly higher in the patient group (8.8 mumol 1(-1) vs. 6.8 mumol 1(-1); p = 0.003). Five patients (20%) had plasma tHcy levels that exceeded the upper normal limit of plasma tHcy (median+2 SD of the reference group). Median S-folate was significantly lower in the patient group (6.0 nmol 1(-1) vs. 8.5 nmol 1(-1); p < 0.001), and 11 (44%) patients had depressed S-folate. Only three (12%) patients had RBC folate values below the reference interval. There was no difference in the levels of RBC folate between the two groups. A comparison of S-cobalamin levels in the two groups disclosed a significantly lower level in the patient group. However, no patient had cobalamin deficiency as assessed by S-cobalamin and S-methylmalonate measurements. Thus, it is unlikely that any patient had increased plasma tHcy due to cobalamin deficiency. Of 24 patients having a HbA1c measurement performed, 12 (50%) had decreased levels indicating chronic haemolysis. Only seven (28%) patients had reticulocytosis. HbA1c was positively correlated to haptoglobin levels (r = 0.77; p < 0.001) and negatively correlated to the percentage of reticulocytes (r = -0.50; p = 0.02). The percentage of reticulocytes was negatively correlated to haptoglobin levels (r = -0.42; p = 0.04). The chronic haemolysis of the patients' blood due to SASP might explain the similar RBC folate values in the two groups because of a relatively higher folate content of young erythrocytes. In conclusion, our results support previous findings of folate deficiency and haemolysis occurring in a considerable fraction of patients receiving treatment with SASP. Measurements of plasma tHcy suggest that a substantial number of patients may have folate deficiency at the tissue level.
Lashner BA, Heidenreich PA, Su GL, Kane SV, Hanauer SB. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis. A case-control study.
Gastroenterology 1989 Aug;97(2):255-259.
Abstract: Folate deficiency has been associated with dysplasia in human cancer models. Patients with ulcerative colitis commonly have decreased folate levels, which are partially due to sulfasalazine, a competitive inhibitor of folate absorption. To study the effect of folate supplementation on the risk of dysplasia or cancer (neoplasia) in ulcerative colitis, records from 99 patients with pancolitis for greater than 7 yr and enrolled in a surveillance program were reviewed. Thirty-five patients with neoplasia were compared with 64 patients in whom dysplasia was never found to determine the effect of folate supplementation on the rate of development of neoplasia using case-control methodology. At the time of the index colonoscopy, patients with neoplasia were older (43 +/- 11 vs. 39 +/- 12 yr) and had disease of longer duration (20 +/- 8 vs. 15 +/- 7 yr, p less than 0.05). Folate supplementation was associated with a 62% lower incidence of neoplasia compared with individuals not receiving supplementation (odds ratio, 0.38; 95% confidence interval, 0.12-1.20). There was no appreciable change in this effect when models were fit to adjust for sulfasalazine dose, duration of disease, age at symptom onset, prednisone dose, sulfa allergy, sex, race, or family history of colon cancer. The statistical power of the association between folate supplementation and neoplasia was 72%. Correction of risk factors before the development of neoplasia may prevent this serious complication. Pending a larger case-control study, folate supplementation during sulfasalazine administration is recommended to possibly prevent the complication of dysplasia or cancer in ulcerative colitis.
Lashner BA, Provencher KS, Seidner DL, Knesebeck A, Brzezinski A. The effect of folic acid supplementation on the risk for cancer or dysplasia in ulcerative colitis.
Gastroenterol 1997 Jan;112(1):29-32 .
Abstract: BACKGROUND & AIMS: Two case-control studies have shown that folate may protect against neoplasia in ulcerative colitis. This historical cohort study was performed to better define this association. METHODS: The records of 98 patients with ulcerative colitis who had disease proximal to the splenic flexure for at least 8 years were reviewed. Documented folate use of at least 6 months was deemed a positive exposure. RESULTS: Of the patients, 29.6% developed neoplasia and 40.2% took folate supplements. The adjusted relative risk (RR) of neoplasia for patients taking folate was 0.72 (95% confidence interval [CI], 0.28-1.83). The dose of folate varied with the risk of neoplasia (RR, 0.54 for 1.0 mg folate; RR, 0.76 for 0.4 mg folate in a multivitamin compared with patients taking no folate). Folate use also varied with the degree of dysplasia (RR for cancer, 0.45; RR for high-grade dysplasia, 0.52; RR for low-grade dysplasia, 0.75 compared with patients with no dysplasia) (P = 0.08). CONCLUSIONS: Although not statistically significant, the RR for folate supplementation on the risk of neoplasia is < 1 and shows a dose-response effect, consistent with previous studies. Daily folate supplementation may protect against the development of neoplasia in ulcerative colitis.
Lindenbaum J. Drugs and vitamin B12 and folate metabolism. Current Concepts in Nutrition 1983;12:73-87. (Review)
Longstreth GF, Green R. Folate levels in inflammatory bowel disease. N Engl J Med 1982 Jun 17;306(24):1488. (Letter)
Longstreth GF, Green R. Folate status in patients receiving maintenance doses of sulfasalazine.
Arch Intern Med 1983 May;143(5):902-904.
Abstract: Hematologic studies, including serum and RBC folate assays, were done on 45 outpatients with chronic colitis who either took sulfasalazine (n = 27) or did not use it (n = 18). Overall, sulfasalazine users and nonusers had similar mean hemoglobin, hematocrit, serum folate, and RBC folate levels. However, within the drug users, RBC folate was inversely correlated with drug dose; serum folate was not. Patients taking 2 g or more of sulfasalazine daily had lower mean RBC folate levels (221.2 +/- 27.3 ng/mL) than patients either taking less (371.7 +/- 35.0 ng/mL) or nonusers (330.3 +/- 30.3 ng/mL). Mean corpuscular volume was also related to drug dose but not to RBC folate. Although maintenance sulfasalazine use rarely causes clinically significant folate deficiency, subclinical tissue depletion occurs as a dose-related effect.
Ma J, Stampfer MJ, Giovannucci E, Artigas C, Hunter DJ, Fuchs C, Willett WC, Selhub J, Hennekens CH, Rozen R. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer.
Cancer Res 1997 Mar 15;57(6):1098-1102.
Abstract: Folate derivatives are important in experimental colorectal carcinogenesis; low folate intake, particularly with substantial alcohol intake, is associated with increased risk. The enzyme 5,10-methylenetetrahydrofolate reductase (MTHFR) catalyzes the conversion of 5,10-methylenetetrahydrofolate, required for purine and thymidine syntheses, to 5-methyltetrahydrofolate, the primary circulatory form of folate necessary for methionine synthesis. A common mutation (677C-->T) in MTHFR reduces enzyme activity, leading to lower levels of 5-methyltetrahydrofolate. To evaluate the role of folate metabolism in human carcinogenesis, we examined the associations of MTHFR mutation, plasma folate levels, and their interaction with risk of colon cancer. We also examined the interaction between genotype and alcohol intake. We used a nested case-control design within the Physicians' Health Study. Participants were ages 40-84 at baseline when alcohol intake was ascertained and blood samples were drawn. During 12 years of follow-up, we identified 202 colorectal cancer cases and matched them to 326 cancer-free controls by age and smoking status. We genotyped for the MTHFR polymorphism and measured plasma folate levels. Men with the homozygous mutation (15% in controls) had half the risk of colorectal cancer [odds ratio (OR), 0.49; 95% confidence interval (CI), 0.27-0.87] compared with the homozygous normal or heterozygous genotypes. Overall, we observed a marginal significant increased risk of colorectal cancer (OR, 1.78; 95% CI, 0.93-3.42) among those whose plasma folate levels indicated deficiency (<3 ng/ml) compared with men with adequate folate levels. Among men with adequate folate levels, we observed a 3-fold decrease in risk (OR, 0.32; 95% CI, 0.15-0.68) among men with the homozygous mutation compared with those with the homozygous normal or heterozygous genotypes. However, the protection due to the mutation was absent in men with folate deficiency. In men with the homozygous normal genotype who drank little or no alcohol as reference, those with the homozygous mutation who drank little or no alcohol had an 8-fold decrease in risk (OR, 0.12; 95% CI, 0.03-0.57), and for moderate drinkers, a 2-fold decrease in risk (OR, 0.42; 95% CI, 0.15-1.20); no decrease in risk was seen in those drinking 1 or more drinks/day. Our findings provide support for an important role of folate metabolism in colon carcinogenesis. In particular, these results suggest that the 677C-->IT mutation in MTHFR reduces colon cancer risk, perhaps by increasing 5,10-methylenetetrahydrofolate levels for DNA synthesis, but that low folate intake or high alcohol consumption may negate some of the protective effect.
Mason JB. Folate and colonic carcinogenesis: Searching for a mechanistic understanding.
J Nutr Biochem 1994;5:170-175.
Mouzas IA, et al. Chemoprevention of colorectal cancer in inflammatory bowel disease? A potential role for folate.
Ital J Gastroenterol Hepatol. 1998 Aug;30(4):421-425. (Review)
Pironi L, Cornia GL, Ursitti MA, Miniero R, Bianchi G, Miglioli M, Barbara L. [Prevalence and pathogenesis of folate deficiency in patients with quiescent or clinically mildly active Crohn disease].
Minerva Dietol Gastroenterol 1987 Oct-Dec;33(4):307-313. [Article in Italian]
Rosenberg IH. Absorption and malabsorption of folates. Clinics in Haematology 1976 Oct;5(3):589-618. (Review)
Selhub J, Dhar GJ, Rosenberg IH. Inhibition of folate enzymes by sulfasalazine.
J Clin Invest 1978 Jan;61(1):221-224.
Swinson CM, Perry J, Lumb M, Levi AJ. Role of sulphasalazine in the aetiology of folate deficiency in ulcerative colitis.
Gut 1981 Jun;22(6):456-461.
Abstract: Only two (2.5%) of 80 outpatients with histologically proven ulcerative colitis had folate deficiency associated with anaemia or macrocytosis. Mean folate absorption, measured using micrograms/kg body weight of a tritium-labelled physiological folate derivative, 5-methyltetrahydroteroylglutamic acid, in six newly diagnosed patients was 76.7% (normal greater than 95%) but fell to 69.4% after three months' treatment with sulphasalazine. Mean difference in individual patients was 7.5% +/- 5.2% (SD) (p less than 0.02). Mean folate absorption in four patients with megaloblastic anaemia or macrocytosis which developed during treatment with sulphasalazine was 66.3%. This rose to 82.4% after the drug was stopped. Mean difference in individual patients was 16.6 +/- 6.6% (SD) (p less than 0.001). All patients who developed anaemia or macrocytosis with sulphasalazine had additional reasons for folate deficiency. These included coeliac disease, severe nutritional deficiencies, and haemolysis. It was concluded that sulphasalazine impairs folate absorption but this only becomes significant if other reasons for folate deficiency are also present.
Threlkeld DS, ed. Gastrointestinal Drugs, Sulfasalazine. In: Facts and Comparisons Drug Information. St. Louis, MO: Facts and Comparisons, Sep 1997.
Weiss, RF. Herbal Medicine. Beaconsfield, England: Beaconsfield Publishers Ltd., 1988.

