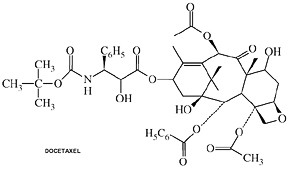

Docetaxel
Brand Names: TaxoterePlease read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
Behar A, Pujade-Lauraine E, Maurel A, Brun MD, Chauvin FF, Feuilhade de Chauvin F, Oulid-Aissa D, Hille D. The pathophysiological mechanism of fluid retention in advanced cancer patients treated with docetaxel, but not receiving corticosteroid
comedication. Br J Clin Pharmacol 1997 Jun;43(6):653-658.
Abstract: AIMS: Fluid retention is a phenomenon associated with taxoids. The principal objective of this study was to investigate the pathophysiological mechanism of docetaxel-induced fluid retention in advanced cancer patients. METHODS: Docetaxel was administered as a 1 h intravenous infusion every 3 weeks, for at least 4-6 consecutive cycles, to patients with advanced breast (n = 21) or ovarian (n = 3) carcinoma, who had received previous chemotherapy, 21 for advanced disease. Phase II clinical trials have shown that 5 day corticosteroid comedication, starting 1 day before docetaxel infusion, significantly reduces the incidence and severity of fluid retention. This prophylactic corticosteroid regimen is currently recommended for patients receiving docetaxel but was not permitted in this study because of its possible interference with the underlying pathophysiology of the fluid retention. RESULTS: Fluid retention occurred in 21 of the 24 patients but was mainly mild to moderate, with only five patients experiencing severe fluid retention. Eighteen patients received symptomatic flavonoid treatment, commonly prescribed after the last cycle. Specific investigations for fluid retention confirmed a relationship between cumulative docetaxel dose and development of fluid retention. Capillary filtration test analysis showed a two-step process for fluid retention generation, with progressive congestion of the interstitial space by proteins and water starting between the second and the fourth cycle, followed by insufficient lymphatic drainage. CONCLUSIONS: A vascular protector such as micronized diosmine hesperidine with recommended corticosteroid premedication and benzopyrones may be useful in preventing and treating docetaxel-induced fluid retention.
Vukelja SJ, Baker WJ, Burris HA 3d, Keeling JH, Von Hoff D. Pyridoxine therapy for palmar-plantar erythrodysesthesia associated with
Taxotere. J Natl Cancer Inst 1993 Sep 1;85(17):1432-1433. (Letter)