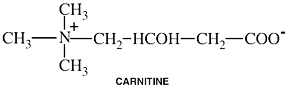

Carnitine
Common Names: Carnitine; L-acetyl-carnitine; Propionyl-L-carnitinePlease read the disclaimer concerning the intent
and limitations of the information provided here.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
![]()
Do not rely solely on the information in this article.
References
Beversdorf D, Allen C, Nordgren R. Valproate induced encephalopathy treated with carnitine in an adult.
J Neurol Neurosurg Psychiatry. 1996 Aug;61(2):211.
Bratton SL, Garden AL, Bohan TP, French JW, Clarke WR. A child with valproic acid-associated carnitine deficiency and carnitine-responsive cardiac dysfunction.
J Child Neurol 1992 Oct;7(4):413-416.
Abstract: Valproic acid enhances renal losses of carnitine esters and leads to decreased plasma free carnitine concentrations in many patients receiving valproic acid therapy. However, decreased serum carnitine levels are of unclear pathologic significance, and most children manifest no symptoms of carnitine deficiency. We report a child with valproic acid-associated carnitine deficiency who had severe cardiac dysfunction develop that resolved with carnitine replacement therapy.
Campos Y, Arenas J. Muscle carnitine deficiency associated with zidovudine-induced mitochondrial
myopathy. Ann Neurol 1994 Oct;36(4):680-681. (Letter)
Castro-Gago M, Camina F, Rodriguezx-Segade S. Carnitine deficiency caused by valproic acid.
J Pediatr 1992 Mar;120(3):496. (Letter)
Castro-Gago M, Eiris-Punal J, Novo-Rodriguez MI, Couceiro J, Camina F, Rodriguez-Segade S. Serum carnitine levels in epileptic children before and during treatment with valproic acid, carbamazepine, and
phenobarbital. J Child Neurol 1998 Nov;13(11):546-549.
Abstract: Serum levels of free, acyl, and total carnitine were determined in 32 patients with seizures, before and after 3, 6, and 12 months of treatment with valproic acid (17 patients), carbamazepine (10 patients), or phenobarbital (5 patients). In all three treated groups, both free and total carnitine levels showed a significant decline with respect to pretreatment levels. This decline was most marked and most consistent in patients treated with valproic acid. In 35% of the patients in this group, carnitine deficiency (ie, total carnitine < 30 micromol/L) was observed by month 12. In none of the three groups were serum carnitine levels significantly correlated with the serum concentration of the drug. These findings suggest a need to monitor serum carnitine levels in children treated with any of these drugs.
Dalakas MC, Leon-Monzon ME, Bernardini I, Gahl WA, Jay CA. Zidovudine-induced mitochondrial myopathy is associated with muscle carnitine deficiency and lipid storage.
Ann Neurol 1994 Apr;35(4):482-487.
Abstract: The use of zidovudine (AZT) for the treatment of acquired immunodeficiency syndrome (AIDS) induces a DNA-depleting mitochondrial myopathy, which is histologically characterized by the presence of muscle fibers with "ragged-red"-like features, red-rimmed or empty cracks, granular degeneration, and rods (AZT fibers). Because dysfunctioning muscle mitochondria may lead to defects of beta-oxidation of fatty acids, we examined the degree of neutral fat accumulation and muscle carnitine levels in the muscle biopsy specimens from 21 patients with AZT-induced myopathic symptoms of varying severity. Six patients with no AZT fibers had normal endomyofibrillar lipid deposits and muscle carnitine levels; 7 patients with fewer than 5 AZT fibers per field had a mild (+) to moderate (++) increase in lipid droplets, and reduced muscle carnitine levels (3 patients); and 8 patients with more than 5 AZT fibers had severe muscle changes, a ++ to marked ( ) increase in lipid droplets, and reduced muscle carnitine levels (6 patients). Serial sections showed lipid globules often within "cracks" or vacuoles of the abnormal muscle fibers. We conclude that the muscle mitochondrial impairment caused by AZT results in (1) accumulation of lipid within the muscle fibers owing to poor utilization of long-chain fatty acids, (2) reduction of muscle carnitine levels probably due to decreased carnitine uptake by the muscle, and (3) depletion of energy stores within the muscle fibers. The findings may have potential therapeutic implications in the treatment of AZT-induced myopathic symptoms using oral carnitine supplementation.
De Vivo DC, Bohan TP, Coulter DL, Dreifuss FE, Greenwood RS, Nordli DR Jr, Shields WD, Stafstrom CE, Tein I. L-carnitine supplementation in childhood epilepsy: current perspectives.
Epilepsia 1998 Nov;39(11):1216-1225.
Abstract: In November 1996, a panel of pediatric neurologists met to update the consensus statement issued in 1989 by a panel of neurologists and metabolic experts on L-carnitine supplementation in childhood epilepsy. The panelists agreed that intravenous L-carnitine supplementation is clearly indicated for valproate (VPA)-induced hepatotoxicity, overdose, and other acute metabolic crises associated with carnitine deficiency. Oral supplementation is clearly indicated for the primary plasmalemmal carnitine transporter defect. The panelists concurred that oral L-carnitine supplementation is strongly suggested for the following groups as well: patients with certain secondary carnitine-deficiency syndromes, symptomatic VPA-associated hyperammonemia, multiple risk factors for VPA hepatotoxicity, or renal-associated syndromes; infants and young children taking VPA; patients with epilepsy using the ketogenic diet who have hypocarnitinemia; patients receiving dialysis; and premature infants who are receiving total parenteral nutrition. The panel recommended an oral L-carnitine dosage of 100 mg/kg/day, up to a maximum of 2 g/day. Intravenous supplementation for medical emergency situations usually exceeds this recommended dosage.
Famularo G, Moretti S, Marcellini S, Trinchieri V, Tzantzoglou S, Santini G, Longo A, De Simone C. Acetyl-carnitine deficiency in AIDS patients with neurotoxicity on treatment with antiretroviral nucleoside analogues.
AIDS 1997 Feb;11(2):185-190.
Abstract: OBJECTIVE: A severe dose limiting axonal peripheral neuropathy may develop in subjects on treatment with the nucleoside analogues didanosine (ddl), zalcitabine (ddC), and stavudine (d4T). The impairment of mitrochondrial DNA synthesis is crucial to the pathogenesis of this disorder although other mechanisms have not been ruled out. The depletion of acetyl-carnitine, which regulates the metabolism and function of peripheral nerves could contribute to the neurotoxicity of these compounds. DESIGN: Non-randomized, cross-sectional study of selected patients. METHODS: We measured the serum levels of acetyl- and total carnitine in 12 subjects with axonal peripheral neuropathy developed on treatment with different regimens of neurotoxic nucleoside analogues (ddl, ddC, d4T). Subjects who did not develop peripheral neuropathy while staying on treatment with ddl (n = 10) or zidovudine (n = 11) served as the control groups. HIV-negative subjects with axonal on demyelinating autoimmune neuropathies (n = 10) and healthy individuals (n = 13) were additional control groups. RESULTS: Subjects experiencing axonal peripheral neuropathy on treatment with ddl, ddC and d4T had significantly reduced levels of acetyl-carnitine in comparison to the control groups. No difference was observed in the levels of total carnitine between study subjects and the control groups. CONCLUSIONS: Our results demonstrate that subjects who developed peripheral neuropathy while staying on treatment with ddl, ddC and d4T had acetyl-carnitine deficiency. The normal levels of total carnitine in the study group appear to indicate the specificity of the defect and rule out coexisting relevant nutritional problems. The critical role of acetyl-carnitine for the metabolism and function of the peripheral nerves supports the view that the acetyl-carnitine deficiency found in these subjects may contribute to the neurotoxicity of ddl, ddC and d4T, even though the interference with mitochondrial DNA synthesis is regarded as the main cause of their toxicity.
Ferro M, Crivello R, Gianotti A, Conti M. [Treatment of hypertrophic cardiomyopathy with a combination of carnitine and beta blockaders. Review of the literature. Description of a clinical case and long-term follow up].
Clin Ter. 1993 Aug;143(2):109-113. (Review) [Article in Italian]
Abstract: In the past decade, strategies for managing heart failure have changed. The use of beta blockers, although still in the experimental stage, has proved effective in some cases. The protective action of beta-blocking agents against chronic catecholamine stimulation may be enhanced by the combination with L-carnitine. This substance plays an important and synergistic role 1) as an important source of energy due to fatty acid oxidation, and 2) by avoiding the accumulation of lipids in the myocardium. The successful follow-up of a case of dilated cardiomyopathy is critically reviewed. Treatment with the L-carnitine-propranolol combination restored cardiac function in a 52-year-old man with dilated cardiomyopathy: a 50% reduction in mitral EPSS (E Point Septal Separation), from 20 to 10 mm was obtained with the above mentioned therapy; as well as a decrease from 60 to 57 mm in diastolic diameter. Our experience suggests promising benefits in adopting beta blockers combined with L-carnitine therapy in myocardial failure secondary to dilated cardiomyopathy.
Freeman JM, Vining EPG, Cost S, Singhi P. Does carnitine administration improve the symptoms attributed to anticonvulsant medications? A double-blinded, crossover study.
Pediatr 1994 Jun;93(6 Pt 1):893-895.
Abstract: OBJECTIVE. This study was designed to assess the reported improvement in "well-being" perceived by parents when children who are taking anticonvulsant medications are administered carnitine. METHODOLOGY. Forty-seven children with seizures who were taking either valproic acid or carbamazepine were enrolled in a placebo-controlled, double-blinded, cross-over study of the effects of oral carnitine administration (100 mg/kilo) on their well-being as perceived by their parents. The well-being scores were assessed weekly by phone and in person at the start and end of each 4-week phase. RESULTS. The children's well-being scores improved weekly when either placebo or carnitine were administered. None of the analyses of improved well-being achieved statistical significance. CONCLUSION. We believe this study documents the necessity for controlled trials when assessing the subjective, beneficial effects of medications. Carnitine is expensive, costing approximately $.30/kilogram of body weight per day ($6 per day for a 20 kilo child). It would not appear warranted to administer carnitine prophylactically to children on anticonvulsant medications for alleviating common, nonspecific symptoms. Because there are no reliable clinical or laboratory tests of symptomatic carnitine deficiency caused by anticonvulsant administration, how to identify children in need of carnitine, and when to administer carnitine therapeutically to children receiving valproate or other anticonvulsants is unclear.
Gidal BE, Inglese CM, Meyer JF, et al. Diet-and valproate-induced transient hyperammonemia: Effect of L-carnitine.
Pediatr Neurol 1997 May;16(4):301-305.
Abstract: Hyperammonemia is an adverse effect of valproate (VPA) treatment. In particular, transient hyperammonemia has been reported to occur in VPA-treated patients after protein-rich meals. This phenomenon may occur secondary to a VPA-mediated carnitine insufficiency. We sought to confirm that protein ingestion would result in transient hyperammonemia and to determine whether supplementation with L-carnitine would prevent this effect. We studied the effect of consumption of a standardized protein-rich meal (45 g protein) before (phase I) and after (phase II) administration of L-carnitine 50 mg/kg/day for 7 days in 11 epileptic children (13.3 +/- 2.3 years of age) receiving VPA. Venous blood was obtained during fasting (baseline) and at 2 and 4 hours after the protein-rich meal for analysis of ammonia (NH3), and VPA concentrations. Mean VPA trough concentrations did not differ significantly at any time. After protein ingestion, 2-hour NH3 concentration increased by 86% (P < .05) from baseline in phase I as compared with a 38% increase in phase II. In both phases I and II, 4-hour NH3 concentrations decreased toward baseline values. We conclude that (1) modest protein ingestion can result in significant transient increases in NH3 in VPA-treated children, (2) significant increases may occur in patients with normal fasting NH3 concentrations, (3) these increases can be significantly attenuated by L-carnitine supplementation, and (4) these changes do not appear to be related to changes in VPA concentration.
Hiraoka A, Arato T, Tominaga I. Reduction in blood free carnitine levels in association with changes in sodium valproate (VPA) disposition in epileptic patients treated with VPA and other anti-epileptic drugs.
Biol Pharm Bull 1997 Jan;20(1):91-93.
Abstract: Reduction in the blood free carnitine (FC) level as a side effect of sodium valproate (VPA) given epileptic patients was pharmacokinetically studied in connection with changes in the VPA disposition. The serum FC level in patients taking at least one of phenobarbital (PB), phenytoin (PHT) and/or carbamazepine (CBZ) in addition to VPA was significantly lower than that in the controls given only these other anti-epileptic drugs (AEDs). Patients medicated only with VPA also tended to have a lower serum FC level than the controls, although the difference was not significant. Among all the patients taking VPA with or without other AED(s), a significantly positive correlation was observed between the serum FC level and the value of dose and level ratio (L/D) of VPA, indicating that both the serum FC concentration and the L/D value of VPA were remarkably reduced in those patients receiving both medications. These results suggested that reduction in the blood FC level as a side effect of VPA reflected FC deficiency associated with the accelerated degradation of VPA in liver; such a condition appears to result from medication with VPA and other AED(s) which induce(s) enzyme(s) for the VPA metabolism.
Hirose S, Mitsudome A, Yasumoto S, Ogawa A, Muta Y, Tomoda Y. Valproate therapy does not deplete carnitine levels in otherwise healthy children.
Pediatrics 1998 May;101(5):E9.
Abstract: OBJECTIVE: To determine whether children with epilepsy undergoing valproate (VPA) antiepileptic therapy and who are otherwise healthy have a lower serum level of carnitine (CAR) and a higher plasma level of plasma ammonia than do normal children. METHODOLOGY: A total of 45 children with epilepsy, 6.3 to 21.7 years of age, who were treated solely with VPA and were free of abnormal neurologic findings or nutritional problems were randomly selected (VPA-treated group). An age-matched control group (n = 45) was selected from subjects without epilepsy (control group). Total (T) and free (F) serum CAR, serum VPA concentration, and the plasma ammonia level were measured and analyzed. RESULTS: Serum VPA concentration exhibited a weak negative correlation with both T- (r = -0.34) and F-CAR (r = -0.41). The T-CAR levels were 55.7 +/- 12.4 and 57.6 +/- 12.1 mM, and the F-CAR levels 42.7 +/- 9.9 and 44.4 +/- 9.9 mM in the VPA-treated and control groups, respectively. Thus, there was no significant difference in T- or F-CAR levels between the VPA-treated and control groups. Plasma ammonia levels were the same in the two groups: 26 +/- 9.2 and 29.4 +/- 11.8 mM in the VPA-treated and control groups, respectively. There was no significant correlation between blood ammonia and either T- (r = +0.024) or F-CAR (r = -0. 026). CONCLUSION: Children on a regular diet ingest a sufficient amount of CAR that more than meets their daily CAR requirement. The level of neither T- nor F-CAR in patients with epilepsy and without severe neurologic or nutritional problems being treated with VPA appeared to be affected by VPA therapy. Because the blood CAR level depends on nutritional condition rather than on blood VPA concentration, CAR deficiency caused by VPA is not likely to occur in this population. The usefulness of supplementation of CAR for this type of patient with epilepsy, therefore, must be reevaluated carefully.
Kelley RI. The role of carnitine supplementation in valproic acid therapy. Pediatr 1994 Jun;93(6 Pt 1):891-892. (Editorial)
Kerns II W, Kline J, Ford MD. Blocker and calcium channel blocker toxicity.
Emerg Med Clinics of NA 1994;12:2:365-390.
Lefkowitz RJ, Hoffman BB, Taylor P: Neurotransmission: The Autonomic and Somatic Motor Nervous Systems, In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG (eds):
Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 9th ed. New York: McGraw-Hill, 1996:105-139.
Marz R. Medical Nutrition From Marz. Second Edition. Portland, OR. 1997.
Murakami K, Sugimoto T, Woo M, Nishida N, Muro H. Effect of L-carnitine supplementation on acute valproate intoxication.
Epilepsia 1996 Jul;37(7):687-689.
Abstract: We analyzed urinary valproate (VPA) metabolites and carnitine concentrations in a child who accidentally ingested 400 mg/kg VPA. The concentration of 4-en VPA, the presumed major factor in VPA-induced hepatotoxicity, was markedly increased, without liver dysfunction or hyperammonemia. The other major abnormality was decreased beta-oxidation and markedly increased omega-oxidation. After L-carnitine supplementation, VPA metabolism returned to normal. The level of valproylcarnitine was not increased and therefore was not affected by L-carnitine. L-Carnitine may be useful in treating patients with coma after VPA overdose.
Murray MT. The many benefits of carnitine. Am J Natural Med 1996;3:6-14. (Review)
Navarro-Quesada FJ, Lluch-Fernandez MD, Vaquero-Abellan M, Marchante-Serrano C, Jimenez C. [Evaluation of the effect of long term valproic acid treatment on plasma levels of carnitine, ammonia and amino acids related to the urea cycle in pediatric epileptic patients]. Rev Neurol 1997 Jul;25(143):1037-1044. [Article in Spanish]
Abstract: INTRODUCTION: Valproic acid (VPA) is an antiepileptic drug widely used in paediatrics. In spite of being a safe and effective anticonvulsant, VPA has been involved in the onset of changes in the metabolism of ammonia and carnitine, although few prospective studies have been made of this. OBJECTIVES: To evaluate the effect of long-term VPA administration, particularly on the metabolism of carnitine, ammonia and plasma amino-acids and the possible clinical repercussions of this in a group of epileptic patients studied prospectively and retrospectively. MATERIAL AND METHODS: A study was made of 102 epileptic children on long term anticonvulsant treatment mainly with VPA. These patients were divided into two groups: group I (n = 25) were studied prospectively (basal sample, after one, six and twelve months of treatment) and group II (n = 77) or long term treatment group (a single sample extraction). In each epileptic patient and in 56 children from a control group (group III) studies were made of free plasma carnitine, ammonia and amino-acids related to the urea cycle and the plasma levels of each anticonvulsant drug. RESULTS: It was observed that in group I there was a fall in plasma carnitine concentrations with time and a progressive rise which was statistically significant (p = 0.001) in plasma levels, mainly of ammonia, glutamine, glycine and ornithine, from the basal levels to those after a year of treatment in practically 100% of the children studied. In group II children on antiepileptic drugs, mainly VPA, were seen to have lower plasma carnitine levels than those in the control group and higher serum ammonia, glutamine and glycine levels than the healthy population not treated with anticonvulsants. These differences were statistically significant (p = 0.001). No relationship was found between the parameters studied and the plasma levels of the drug, type of epilepsy or presence of side effects. CONCLUSIONS: These changes show the negative effects of VPA on the metabolism of carnitine and ammonia. It would therefore seem advisable to monitor these parameters in epileptic children on long term antiepileptic treatment.
Pepine CJ. The therapeutic potential of carnitine in cardiovascular disorders.
Clin Ther. 1991 Jan-Feb;13(1):2-21; discussion 1. (Review)
Abstract: The naturally occurring compound L-carnitine plays an essential role in fatty acid metabolism. It is only by combining with carnitine that the activated long-chain fatty acyl coenzyme A esters in the cytosol are able to be transported to the mitochondrial matrix where beta-oxidation occurs. Carnitine also functions in the removal of compounds that are toxic to metabolic pathways. Clinical evidence indicates that carnitine may have a role in the management of a number of cardiovascular disorders. Supplemental administration of carnitine has been shown to reverse cardiomyopathy in patients with systemic carnitine deficiency. Experimental evidence obtained in laboratory animals and the initial clinical experience in man indicate that carnitine may also have potential in the management of both chronic and acute ischemic syndromes. Peripheral vascular disease, congestive heart failure, cardiac arrhythmias, and anthracycline-induced cardiotoxicity are other cardiovascular conditions that may benefit from carnitine administration, although at this time data on the use of carnitine for these indications are very preliminary.
Prasad K. Homocysteine, a Risk Factor for Cardiovascular Disease. Intl J Angiology 1999 Jan;8(1):76-86.
Abstract: Fasting hyperhomocysteinemia is an independent risk factor for coronary artery disease, stroke, peripheral vascular atherosclerosis, and for arterial and venous thromboembolism. The risk for cardiovascular disease with homocysteine is similar to conventional risk factors. The interaction of hyperhomocysteinemia with hypertension and smoking is strong and the combined effect is more than multiplicative. The combined effect of homocysteine and cholesterol is additive. Homocysteine produces atherosclerosis, thromboembolism, and vascular endothelial cell injury. Vascular dysfunction produced by homocysteine may be due to endothelial cell damage. Homocysteinemia-induced atherosclerosis is probably due to various factors including endothelial cell injury, inability to sustain S-nitroso-homocysteine formation because of imbalance between production of nitric oxide by dysfunctional endothelium and homocysteine, smooth muscle cell proliferation, and thromboembolism. There is strong evidence that endothelial cell injury is associated with oxidative stress produced by homocysteine. Hyperhomocysteinemia is associated with numerous conditions, including coronary disease, stroke, peripheral vascular disease (carotid artery and cerebrovascular atherosclerosis), venous thrombosis, renal disease, diabetes mellitus, and organ transplant. Folic acid, vitamin B12 and B6 have been shown to be beneficial in reducing plasma homocysteine levels. Folic acid is specifically very effective, safe and inexpensive.
Raby WN. Carnitine for valproic acid-induced hyperammonemia. Am J Psychiatry. 1997 Aug;154(8):1168-1169.
Sakemi K, Takada G. Effect of carnitine on valproic acid concentrations in serum, brain, and liver.
Pediatr Neurol 1998 Apr;18(4):331-333.
Abstract: This study investigates the influence of L-carnitine supplementation on valproic acid concentrations in rat serum, brain, and liver. Carnitine supplementation increased carnitine concentrations significantly in serum and liver but not in the brain. Free valproic acid concentrations in the brain were significantly increased by carnitine supplementation without any change of carnitine concentrations in the brain. The increase of serum-free valproic acid concentrations by carnitine supplementation apparently caused brain-free valproic acid concentrations to increase. This study suggests that L-carnitine supplementation to valproic acid therapy may potentiate valproic acid effects in the brain, even when the clinical dosage in humans is used.
Semino-Mora MC, Leon-Monzon ME, Dalakas MC. Effect of L-carnitine on the zidovudine-induced destruction of human myotubes. Part I: L-carnitine prevents the myotoxicity of AZT in vitro.
Lab Invest 1994 Jul;71(1):102-112.
Abstract: BACKGROUND: Zidovudine (AZT) as used in the treatment of AIDS, causes a mitochondrial myopathy characterized by depletion of mitochondrial DNA, enzymatic defects in the respiratory chain system, and accumulation of lipid droplets. Most of these changes are also seen in normal human myotubes treated with AZT. Because L-carnitine plays a major role in the transport of long chain fatty acids across the inner mitochondrial membrane and facilitates the beta-oxidation of fatty acids, we examined the effect of L-carnitine in preventing the destructive effect of AZT on the mitochondria and the myotubes of human muscle in tissue culture. EXPERIMENTAL DESIGN: Myotubes, prepared from human muscle biopsies, were exposed to various concentrations of AZT for up to 3 weeks. One-third of the flasks were treated with AZT alone, another third with AZT plus L-carnitine and another third were untreated. The cultures were evaluated with: (a) immunocytochemistry counting the number of myotubes stained with antibodies to Leu-19; (b) enzyme histochemistry for NADH reaction and oil-red-O stain to assess mitochondrial enzymatic activity and lipid droplet accumulation; and (c) electron microscopy counting all the organelles within representative sections of the myotubes, at x24,000, and calculating the volumetric density of each organelle/unit volume of tissue. RESULTS: AZT, at concentrations 250 microM and above, caused depopulation of the Leu-19-positive myotubes, destructive changes in the mitochondria consisting of swelling, lamellar inclusions and multiple concentric cristae, accumulation of lipid droplets, and increase lysosomes. L-Carnitine increased the number of Leu-19-positive myotubes from 3.4 +/- 0.6 to 9.4 +/- 1.2, preserved the morphology of the mitochondria, increased their volumetric density from 2.5 +/- 0.4 to 6.0 +/- 0.7, and reduced the volumetric density of the lipid droplets from 12.2 +/- 4.9 to 1.4 +/- 0.7 and of the lysosomes from 15.6 +/- 3.6 to 3.9 +/- 1.4 (p < 0.001). CONCLUSIONS: L-Carnitine, used concurrently with AZT, prevents the human myotubes from the AZT-associated destruction, preserves the structure and volume of mitochondria and prevents the accumulation of lipids. The findings may have potential clinical implications in preventing the myotoxicity of AZT in patients with AIDS.
Stadler DD, Bale JF Jr, Chenard CA, Rebouche CJ. Effect of long-term valproic acid administration on the efficiency of carnitine reabsorption in humans.
Metabolism 1999 Jan;48(1):74-79.
Abstract: To elucidate the etiology of valproic acid-induced carnitine deficiency, we tested the hypothesis that long-term valproic acid administration decreases the rate of carnitine reabsorption. Thirteen healthy men participated in a 34-day protocol in which carnitine clearance was measured before and after 28 days of valproic acid administration. During valproic acid administration (days 6 to 33), plasma free and total carnitine concentrations decreased (18% and 12%, respectively, P<.05) by 16 days, but returned to pretreatment concentrations by 28 days. From day 14 to day 30, the rate of free carnitine excretion was 50% lower than at baseline (day 4, P<.05). Free and total carnitine clearance, indexed to the glomerular filtration rate, was lower after valproic acid administration (P<.01). Contrary to our hypothesis, after 28 days of valproic acid administration, the rate of carnitine reabsorption was enhanced independent of the glomerular filtration rate and filtered load. Changes in the plasma concentration, rate of excretion, and clearance were specific for carnitine and were not generalized in magnitude or direction to the other amino acids. We conclude that the kidney adapts to conserve carnitine during valproic acid administration and therefore does not cause valproic acid-induced carnitine depletion in adults.
Triggs WJ, Gilmore RL, Millington DS, Cibula J, Bunch TS, Harman E. Valproate-associated carnitine deficiency and malignant cerebral edema in the absence of hepatic failure.
Int J Clin Pharmacol Ther 1997 Sep;35(9):353-356.
Van Wouwe JP. Carnitine deficiency during valproic acid treatment. Internat J Vit Nutr Res
1995;65(3):211-214.
Abstract: Prolonged valproic acid treatment results in secondary carnitine deficiency. In thirteen children paired samples of plasma were drawn at the onset of, and after 9 months of continuous valproic acid treatment. At onset free plasma carnitine values were age dependent; they increased during childhood (r = 0.59, p = 0.016). After 9 months: 1 - mean plasma free carnitine decreased by 40%, from 32.7 mumol/l to 20.9 (p 0.0008 and 3% overlap; 2 - plasma total carnitine decreased by 20%, from 34.9 mumol/l to 27.1 (p 0.016 and no overlap); and 3 - the esterified/free carnitine ratio increased by 40%, from 0.28 to 0.39 (p 0.011 and no overlap). In two out of thirteen patients clinical symptoms were observed, fatigue, besides the biochemical evidence of carnitine deficiency. In four others only biochemical deficiency was found. If the child complains of fatigue during prolonged valproic acid treatment, it is advised to supplement carnitine. A dose of 15 mg/kg body weight is effective to reverse the clinical symptoms of carnitine deficiency within a week. The dose to prevent deficiency is not yet established.
Vikre-Jorgensen J. [Cardiomyopathy caused by carnitine deficiency]. Ugeskr Laeger.
1993 Oct 18;155(42):3390-3392. [Article in Danish]
Abstract: Primary carnitine deficiency often presents as progressive cardiomyopathy. It is due to a defect in the plasma membrane carnitine transport system that is normally present in heart, muscle and kidney. This system serves to maintain intracellular carnitine levels 20-50 times higher than plasma concentrations. Patients with this defect cannot maintain adequate carnitine levels in muscle tissue for fatty acid oxidation. One case of primary carnitine deficiency is described. Two siblings had died earlier probably due to the same disease. The eight month old boy presented with a common cold and cardiomyopathy. He was treated with digoxin and diuretics until the diagnosis was confirmed. The patient's uptake of carnitine in fibroblasts was extremely low, about 5% of the normal range. The father had about 50% reduction of carnitine uptake in fibroblasts, the mother showed no sign of impaired uptake. The boy was treated with oral carnitine, 100 mg/kg/day. There was a normal level of carnitine in serum after two months of treatment and the cardiomyopathy disappeared completely in one year. Primary carnitine deficiency is a treatable disorder and therefore skeletal muscle biopsy and blood chemistry should be performed in all children with undiagnosed cardiomyopathy. Treatment with oral carnitine must be initiated quickly to avoid sudden death.
Werbach MR. Foundations of Nutritional Medicine. Tarzana, CA: Third Line Press, 1997. (Review).
Wolf LR. Adrenergic Blocker Toxicity, in Haddad L, Shannon MW, Winchester JF (eds):
Clinical Management of Poisoning and Drug Overdose, 3rd ed. Pennsylvania: WB Sanders Co, 1998:1031-1040.
Zelnik N, Fridkis I, Gruener N. Reduced carnitine and antiepileptic drugs: cause relationship or co-existence?
Acta Paediatr 1995 Jan;84(1):93-95.
Abstract: Serum carnitine was measured longitudinally before and after therapy in 15 patients receiving valproic acid, 14 patients receiving carbamazepine and 8 patients receiving phenobarbital. The patients who received valproic acid showed a significant reduction in free (and total) serum carnitine (mean (SE) 37.6 (6.2) mumol/l without valproic acid, 29.1 (1.6) mumol/l with valproic acid (p < 0.001). Such an effect was not found in patients receiving carbamazepine or
phenobarbital.