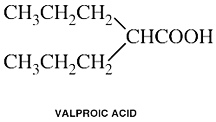
Valproic Acid
Brand Names: Depakene, Depakote
Clinical Names: Divalproex Sodium, Valproic Acid, Valproate
Summary
generic name: Valproic Acid
trade name: Depakene®
related drugs and trade names:
• Divalproex Sodium: Depakote®
• Sodium Valproate: Depakene® Syrup
type of drug: Primarily anticonvulsant; also antimanic, migraine headache prophylactic
used to treat:
• Valproic Acid, Valproate Sodium: anticonvulsant, for seizure disorders such as epilepsy
• Divalproex: anticonvulsant, antimanic, migraine headache prophylactic
Note: Divalproex and valproate sodium form valproic acid in the body.
overview of interactions:
• nutrients affecting drug toxicity: Vitamins, in general
• nutrient affecting drug toxicity: Antioxidants, especially Vitamin E
(Alpha-tocopherol) and Selenium
• nutrient affecting drug performance: Vitamin B6
(Pyridoxine)
• nutrient affected by drug: Vitamin B12
(Cobalamin)
• nutrient affected by drug: Folic Acid
(Folate)
• nutrient affected by drug: Carnitine
(L-carnitine)
• nutrient affected by drug: Copper
• nutrient affected by drug: Selenium
• nutrient affected by drug: Zinc
• diet affecting drug toxicity: Food
• substance affecting drug toxicity: Alcohol
Interactions
nutrients affecting drug toxicity: Vitamins, in general
• mechanism: Valproic acid may cause increased excretion rates of many nutrients as well as other metabolic abnormalities. This can especially be a major concern in pregnant women where the risk of drug-induced birth defects can be significant.
• reports: Baggot et al and other researchers have reported that during multivitamin supplementation, many previously increased excretion rates decreased significantly and fetal head growth often improved.
(Baggot PJ, et alt. Epilepsia 1999 Apr;40(4):512-515; Jurima-Romet M, et al.
Toxicology 1996 Aug 1;112(1):69-85.)
• nutritional support: Individuals taking valproic acid might benefit from supplementation with a multivitamin formulation. These benefits can be particularly critical among pregnant women using valproate. Individuals taking valproic acid should consult their prescribing physician and/or a nutritionally trained healthcare professional about the potential use of a multivitamin supplement to counter the drug's adverse effects.
nutrient affecting drug toxicity: Antioxidants, especially Vitamin E (Alpha-tocopherol) and Selenium
• mechanism: Specific oxidative metabolites of valproic acid have been associated with the drug's toxicity.
• research: Research indicates that the valproic acid's cytotoxic activity is the result of generation of hydrogen peroxide and the production of highly reactive hydroxyl free radicals. Graf et al looked at children with serious adverse experiences with valproic acid and found that glutathione peroxidase was significantly depressed and glutathione reductase was significantly elevated relative to other subjects. In particular, they reported that selenium and zinc concentrations were lower in serious adverse experience patients than in controls and concluded that selenium dependent antioxidant activity might play a special role protecting against adverse reactions. Buchi et al found that the free-radical scavenging action of alpha-tocopherol (vitamin E) and N,N'-diphenyl-p-phenylenediamine (DPPD) protected against lipid peroxidation and hepatotoxicity caused by valproic acid (VPA) in rats.
(Nurge ME, et al. Nutr Res 1991;11:949-960; Tabatabaei AR, et al. Toxicology 1996 Aug 1;112(1):69-85; Tabatabaei AR, Abbott FS.
Chem Res Toxicol 1999 Apr;12(4):323-330; Graf WD, et al. Neuropediatrics 1998 Aug;29(4):195-201; Buchi KN, et al.
J Clin Pharmacol 1984 Apr;24(4):148-154.)
• nutritional support: While the use of valproic acid produces numerous adverse effects, especially upon the liver, no conclusive evidence has emerged to demonstrate the clinical role of vitamin E and selenium, or other antioxidants, in countering these adverse effects. Individuals taking valproic acid should consult their prescribing physician and/or a nutritionally trained healthcare professional regarding the use of antioxidants as part of a nutritional support program.
nutrient affecting drug performance: Vitamin B6
(Pyridoxine)
• research: Ito et al studied the effects of high doses of vitamin B6, valproic acid, or both on twenty patients with infantile spasms and concluded that the combination of vitamin B6 and valproic acid was effective and safe in the treatment of infantile spasms.. Their research found that while vitamin B6 alone provided some benefit, patients who were given a combination of vitamin B6 and valproic acid had significantly fewer seizures and better electroencephalograms than did the group treated initially with vitamin B6 alone. Pietz et al found that 300 mg/kg/day of vitamin B6 (pyridoxine-HCl, orally) reduced infantile spasms in 5 of 17 children within the first two weeks of treatment and within 4 weeks all five patients were free of seizures.
(Ito M, et al. Pediatr Neurol 1991 Mar-Apr;7(2):91-96; Pietz J, et al. Epilepsia 1993 Jul-Aug;34(4):757-763.)
Apart from its emerging reputation as a risk factor in cardiovascular disease homocysteine has been used as an experimental convulsant. Several researchers have found elevated levels of homocysteine in epileptics using anticonvulsant medications but at this point evidence is mixed as to whether this is attributable to the underlying disease process, the medication, or both. The clinical implications of this potential interaction are uncertain at this time. However, supplementation with vitamin B6, vitamin B12, or folate has been considered as offering therapeutic potential since nutritional deficiencies of these nutrients are associated with increased homocysteine plasma concentrations.
(Schwaninger M, et al. Epilepsia 1999 Mar;40(3):345-350.)
• nutritional synergy: Supplemental use of vitamin B6 at high dosages (200 mg or more per day) carries known risks, including eventual damage to sensory nerves. Individuals taking valproic acid should consult their prescribing physician about possible benefit and risks from the simultaneous use of vitamin B6 and only undertake such use under supervision.
nutrient affected by drug: Vitamin B12
(Cobalamin)
• mechanism: In general, anticonvulsants may cause vitamin B12 depletion or deficiency.
• nutritional support: While vitamin B12 is generally considered nontoxic, individuals taking valproic acid should consult their prescribing physician about supplementation and only undertake such use under supervision. Serum and erythrocyte folate levels and hematological profiles should be obtained at regular intervals and adequate folic acid should be administered to overcome the increased folate requirement.
(Roe DA. 1985: 245-259.)
nutrient affected by drug: Folic Acid
(Folate)
• research: Valproic acid, divalproex and valproate sodium have all been reported to cause birth defects when taken during the first 3 months of pregnancy. Specifically, the use of valproic acid during early pregnancy can result in a 1-2% incidence of spina bifida aperta, a closure defect of the posterior neural tube in the human. Hendel et al conducted research on the effect of carbamazepine and valproate treatment on folate metabolism in eleven epileptic patients and interpreted their findings as an inhibition of intestinal folic acid absorption caused by the antiepileptic therapy. However Kishi et al the role of induction of liver enzymes by antiepileptic drugs in folate depletion and determined that patients treated with valproate, a non-enzyme-inducer, exhibited serum folate levels that did not differ significantly from values in controls. Furthermore, neural tube defects in humans and rodents associated with valproic acid do not seem to be folate-deficiency related and their incidence has not changed with administration of folic acid or its derivative, folinic acid. Overall, several other studies have indicated that valproic acid has the least antifolate action of the major anticonvulsant medications and that folate is probably not involved in the mechanism of VPA-induced embryotoxicity. It might also be noted that one study found that the consumption of ethanol potentiated valproic acid-induced neural tube defects in mice due to toxicokinetic interactions.
(Hansen DK, et al. Teratology 1995 Nov;52(5):277-285; Goggin T, et al. Q J Med
1987 Nov;65(247):911-919; Hendel J, et al. Acta Neurol Scand 1984 Apr;69(4):226-231; Elmazar MM, Nau H.
Reprod Toxicol 1995 Sep-Oct;9(5):427-433.)
The use of valproic acid has often been found to be associated with decreased folate levels, as well as a related elevation in plasma concentrations of homocysteine. Apart from its emerging reputation as a risk factor in cardiovascular disease homocysteine has been used as an experimental convulsant. Several researchers have found elevated levels of homocysteine in epileptics using anticonvulsant medications but at this point evidence is mixed as to whether this is attributable to the underlying disease process, the medication, or both. However, the clinical implications of this potential interaction are uncertain at this time.
(Schwaninger M, et al. Epilepsia 1999 Mar;40(3):345-350.)
• nutritional concerns: While folate is generally considered nontoxic, large doses of folic acid may precipitate clinical B12 deficiency, especially if vitamin B12 status was already impaired. Individuals taking valproic acid should consult their prescribing physician about assessment of their serum B12 levels and only undertake supplementation of folate under supervision.
(Roe DA. 1985:245-259.)
nutrient affected by drug: Carnitine (L-carnitine)
• mechanism: Prolonged treatment with valproic acid, more than other anticonvulsants, enhances renal losses of carnitine esters, lowers serum carnitine levels, and results in secondary carnitine deficiency. In most cases these decreased carnitine levels have no obvious pathologic significance, and most children manifest no symptoms of carnitine deficiency. However, they may occasionally cause symptoms of carnitine deficiency such as severe cardiac dysfunction. In a related development, use of carnitine has emerged as a treatment for the acute valproic acid toxicity.
(Bratton SL, et al. J Child Neurol 1992 Oct;7(4):413-416; Nurge ME, et al.
Nutr Res 1991;11:949-60; Kelley RI. Pediatr 1994;93:891-892; Van Wouwe JP.
Internat J Vit Nutr Res 1995;65(3):211-214; Zelnik N, et al. Acta Paediatr 1995 Jan;84(1):93-95; Hiraoka A, et al.
Biol Pharm Bull 1997 Jan;20(1):91-93; Murakami K, Sugimoto T, Woo M, et al.
Epilepsia 1996 Jul;37(7):687-689; Beversdorf D, et al. J Neurol Neurosurg Psychiatry. 1996 Aug;61(2):211.)
• research: Van Wouwe reported that children who complained of fatigue during prolonged valproic acid treatment experienced a reversal of these clinical symptoms of carnitine deficiency within a week of initiating carnitine supplementation at 15 mg/kg of body weight. According to Gidal et al, dosages of carnitine supplementation, 50 mg per 2.2 pounds of body weight, have been found to protect children from increases in blood levels of ammonia due to valproic acid. However, in a double-blind, crossover study of children treated with valproic acid, Freeman et al found that carnitine supplementation, at levels of 100 mg per 2.2 pounds of body weight, was not significantly more effective than placebo at promoting enhanced sense of well-being.
Furthermore, recent research published by Sakemi and Takada indicated L-carnitine supplementation to valproic acid therapy may potentiate valproic acid. They reported that L-carnitine supplementation increased carnitine concentrations significantly in serum and liver but not in the brain and that the resultant increase of serum-free valproic acid concentrations by carnitine supplementation apparently caused brain-free valproic acid concentrations to increase. Overall, the research literature reveals understanding that carnitine has an effect on the performance and toxicity of valproic acid but no clear agreement as to a potential therapeutic role for carnitine has emerged.
(Van Wouwe JP. Internat J Vit Nutr Res 1995;65(3):211-214; Gidal BE, et al.
Pediatr Neurol 1997 May;16(4):301-305; Castro-Gago M, et al. J Child Neurol 1998 Nov;13(11):546-549; Raby WN.
Am J Psychiatry. 1997 Aug;154(8):1168-1169; Zelnik N, et al. Acta Paediatr 1995 Jan;84(1):93-95; De Vivo DC, et al.
Epilepsia 1998 Nov;39(11):1216-122; Triggs WJ, et al. Int J Clin Pharmacol Ther 1997 Sep;35(9):353-356; Stadler DD, et al.
Metabolism 1999 Jan;48(1):74-79; Navarro-Quesada FJ, et al. Rev Neurol 1997 Jul;25(143):1037-1044; Hirose S, et al.
Pediatrics 1998 May;101(5):E9;
Sakemi K, Takada G.et al. Pediatr Neurol 1998 Apr;18(4):331-333.)
• nutritional support: Carnitine deficiency due to valproic acid may be corrected with carnitine supplementation. However, no consensus has emerged as to the clinical value of carnitine supplementation for individuals taking valproic acid. Individuals taking valproic acid should consult their prescribing physician before beginning to use carnitine and only proceed with such supplementation under supervision.
(Castro-Gago M, et al. J Child Neurol 1998 Nov;13(11):546-549; Sakemi K, Takada G.et al.
Pediatr Neurol 1998 Apr;18(4):331-333.)
nutrient affected by drug: Copper
• research: In several studies of children with epilepsy being treated with valproic acid serum copper levels remained unchanged relative to control groups.
(Sozuer DT, et al. J Basic Clin Physiol Pharmacol 1995;6(3-4):265-269; Lerman-Sagie T, et al.
Clin Neuropharmacol 1987;10(1):80-86; Hurd RW, et al. Neurology 1984 Oct;34(10):1393-1395.)
However, Kaji et al found that patients treated with valproic acid, alone or in combination with other drugs, had significantly lower levels of serum copper than did normal controls. These researchers suggested that physicians should watch for potential symptoms of copper deficiency even none of their patients showed any symptoms of copper deficiency at the time of the study.
(Kaji M, et al. Epilepsia 1992 May-Jun;33(3):555-557.)
• nutritional support: No definitive evidence has emerged as to the prevalence or clinical significance of copper depletion or deficiency as a result of valproic acid. Individuals taking valproic acid should consult their prescribing physician and/or a nutritionally trained healthcare professional about the potential use of a copper supplement to restore proper trace mineral balance. Inadequate consumption of copper through dietary sources is common. Supplementation with copper at levels of 1-3 mg per day may be beneficial, being especially for anyone taking zinc supplements.
nutrient affected by drug: Selenium
• research: In 1984 Hurd et al published findings which showed that administration of valproic acid for one week produced significant depletion of zinc and selenium in plasma of rats and a one-third reduction of hepatic selenium. They also noted that patients who were treated chronically with valproic acid as their sole anticonvulsant medication had decreased plasma selenium levels. More recently, Graf et al looked at children with serious adverse experiences with valproic acid and found that glutathione peroxidase was significantly depressed and glutathione reductase was significantly elevated relative to other subjects. In particular, they reported that selenium and zinc concentrations were lower in serious adverse experience patients than in controls and concluded that selenium dependent antioxidant activity might play a special role protecting against adverse reactions.
(Hurd RW, et al. Neurology 1984 Oct;34(10):1393-1395; Graf WD, et al. Neuropediatrics 1998 Aug;29(4):195-201.)
Note: See also discussion of Antidoxidants above.
• nutritional support: Preliminary evidence indicates a pattern of selenium deficiency associated with valproic acid even though there may be no consensus as to its prevalence or clinical significance. Nevertheless, inadequate presence of selenium in dietary sources is common, especially in areas where soils are selenium deficient. Supplementation with selenium at levels of 100-200 mcg per day may be beneficial. Individuals taking valproic acid should consult their prescribing physician and/or a nutritionally trained healthcare professional about the potential use of a selenium supplement to restore proper trace mineral balance.
nutrient affected by drug: Zinc
• research: In 1984 Hurd et al published findings which showed that administration of valproic acid for one week produced significant depletion of zinc, as well as selenium, in the plasma of rats. Sozuer et al found that patients treated with valproic acid, alone or in combination with other drugs, had significantly lower levels of serum zinc than did normal controls.
(Hurd RW, et al. Neurology 1984 Oct;34(10):1393-1395; Sozuer DT, et al. J Basic Clin Physiol Pharmacol
1995;6(3-4):265-269.)
However, In at least two subsequent studies of children with epilepsy being treated with valproic acid serum zinc levels remained unchanged relative to control groups. Kaji et al noted that this finding contrasted with previously reported results of animal experiments.
(Lerman-Sagie T, et al. Clin Neuropharmacol 1987;10(1):80-86; Kaji M, et al.
Epilepsia 1992 May-Jun;33(3):555-557.)
• nutritional support: No definitive evidence has emerged as to the prevalence or clinical significance of zinc depletion or deficiency associated with valproic acid. Dietary sources of zinc are often inadequate, even though common foods such as black-eyed peas, eggs, meat, oysters, seafood, tofu and wheat germ are rich in zinc. Supplementation with zinc, at levels of 20-40 mg three times per day, may also be beneficial. Individuals taking valproic acid should consult their prescribing physician and/or a nutritionally trained healthcare professional about the potential use of supplemental zinc to restore proper trace mineral balance. Anyone adding zinc to their therapeutic regime will also want to supplement with copper to prevent zinc-induced deficiencies of that mineral.
diet affecting drug toxicity: Food
• nutritional support: Patients taking the oral dosage forms of valproic acid and divalproex are often advised to take their medications with meals or snacks to reduce stomach upset. Likewise, patients taking the syrup form of valproic acid may find it more palatable if they mixed it with a liquid or add it to food. Generally physicians and pharmacists recommend that these drugs be swallowed whole, rather than chewed, when taking them in the form of capsules, tablets, or sprinkles to reduce the risk of mouth and throat irritation.
(Threlkeld DS, ed. May 1997.)
substance affecting drug toxicity: Alcohol
• research: Alcohol and valproic acid interact synergistically to reduce mental clarity and coordination and increase drowsiness and dizziness. An increased risk of accidental injury can result from consuming alcohol while taking valproic acid. Elmazar and Nau have also published findings showing that the combination of ethanol and valproic acid can induce neural tube defects in mice due to toxicokinetic interactions.
(Threlkeld DS, ed. May 1997; Elmazar MM, Nau H. Reprod Toxicol 1995 Sep-Oct;9(5):427-433.)
• dietary concerns: Individuals taking any form of valproic acid should avoid consuming alcohol.


Please read the disclaimer concerning the intent
and limitations of the information provided here.
Do not rely solely on the information in this article.
The information presented in Interactions is for
informational and educational purposes only. It is based on scientific
studies (human, animal, or in vitro), clinical experience, case
reports, and/or traditional usage with sources as cited in each
topic. The results reported may not necessarily occur in all
individuals and different individuals with the same medical conditions
with the same symptoms will often require differing treatments. For
many of the conditions discussed, treatment with conventional medical
therapies, including prescription drugs or over-the-counter
medications, is also available. Consult your physician, an
appropriately trained healthcare practitioner, and/or pharmacist for
any health concern or medical problem before using any herbal products
or nutritional supplements or before making any changes in prescribed
medications and/or before attempting to independently treat a medical
condition using supplements, herbs, remedies, or other forms of
self-care.
References
Baggot PJ, Kalamarides JA, Shoemaker JD. Valproate-induced biochemical abnormalities in pregnancy corrected by vitamins: a case report.
Epilepsia 1999 Apr;40(4):512-515.
Abstract: PURPOSE: Valproate (VPA) is a teratogenic anticonvulsant (AED), but vitamin supplementation has been suggested to limit the effect of VPA on the fetus. Maternal urinary metabolites were monitored to assess the metabolic effects of VPA before and after vitamin supplementation. METHODS: A pregnant woman with epilepsy receiving VPA and ethosuximide (ESM) was given high-dose multivitamins from 13 to 28 weeks' gestation. Maternal urinary metabolites were measured throughout the pregnancy by gas chromatography/mass spectrometry. RESULTS: Before multivitamin supplementation began, the patient had significantly increased excretion rates of alpha-ketoglutarate, beta-lactate, pyruvate, lactate, methylmalonate, and other metabolites compared with normal pregnant women. During multivitamin supplementation, many previously increased excretion rates decreased significantly. Fetal head growth was normal up to 30 weeks, but then lagged. Bitemporal narrowing was noted at birth. CONCLUSIONS: VPA may cause metabolic abnormalities in pregnancy. Many biochemical abnormalities attributable to VPA in this patient were corrected with high-dose multivitamin supplementation. The specific relation between biochemical abnormalities and VPA teratogenesis remains to be determined.
Beversdorf D, Allen C, Nordgren R. Valproate induced encephalopathy treated with carnitine in an adult.
J Neurol Neurosurg Psychiatry. 1996 Aug;61(2):211.
Bratton SL, Garden AL, Bohan TP, French JW, Clarke WR. A child with valproic acid-associated carnitine deficiency and carnitine-responsive cardiac dysfunction.
J Child Neurol 1992 Oct;7(4):413-416.
Abstract: Valproic acid enhances renal losses of carnitine esters and leads to decreased plasma free carnitine concentrations in many patients receiving valproic acid therapy. However, decreased serum carnitine levels are of unclear pathologic significance, and most children manifest no symptoms of carnitine deficiency. We report a child with valproic acid-associated carnitine deficiency who had severe cardiac dysfunction develop that resolved with carnitine replacement therapy.
Buchi KN, Gray PD, Rollins DE, Tolman KG. Protection against sodium valproate injury in isolated hepatocytes by alpha-tocopherol and N,N'-diphenyl-p-phenylenediamine.
J Clin Pharmacol 1984 Apr;24(4):148-154.
The possibility that lipid peroxidation is involved in valproic acid (VPA) hepatotoxicity was explored by testing the ability of the free-radical scavengers alpha-tocopherol (vitamin E) and N,N'-diphenyl-p-phenylenediamine (DPPD) to protect against VPA toxicity. Rat hepatocyte cultures were treated with toxic doses of VPA, in conjunction with varying doses of vitamin E and DPPD. Lactate dehydrogenase (LDH) release into the culture media was used to calculate an LDH index as a measure of toxicity. Vitamin E afforded increasing protection against VPA toxicity at concentrations of 1.0 to 4.0 microM but then leveled off and did not give complete protection at concentrations up to 8.0 microM. No protection was seen at less than 1.0 microM. DPPD showed increasing protection from 0.05 to 0.50 microM, with complete protection at the highest concentration. These data indicate that VPA toxicity can be prevented by simultaneous administration of free-radical scavengers and support the concept that VPA hepatotoxicity is due to lipid peroxidation.
Castro-Gago M, Camina F, Rodriguezx-Segade S. Carnitine deficiency caused by valproic acid.
J Pediatr 1992 Mar;120(3):496. (Letter)
Castro-Gago M, Eiris-Punal J, Novo-Rodriguez MI, Couceiro J, Camina F, Rodriguez-Segade S. Serum carnitine levels in epileptic children before and during treatment with valproic acid, carbamazepine, and phenobarbital.
J Child Neurol 1998 Nov;13(11):546-549.
Abstract: Serum levels of free, acyl, and total carnitine were determined in 32 patients with seizures, before and after 3, 6, and 12 months of treatment with valproic acid (17 patients), carbamazepine (10 patients), or phenobarbital (5 patients). In all three treated groups, both free and total carnitine levels showed a significant decline with respect to pretreatment levels. This decline was most marked and most consistent in patients treated with valproic acid. In 35% of the patients in this group, carnitine deficiency (ie, total carnitine < 30 micromol/L) was observed by month 12. In none of the three groups were serum carnitine levels significantly correlated with the serum concentration of the drug. These findings suggest a need to monitor serum carnitine levels in children treated with any of these drugs.
De Vivo DC, Bohan TP, Coulter DL, Dreifuss FE, Greenwood RS, Nordli DR Jr, Shields WD, Stafstrom CE, Tein I. L-carnitine supplementation in childhood epilepsy: current perspectives.
Epilepsia 1998 Nov;39(11):1216-1225.
Abstract: In November 1996, a panel of pediatric neurologists met to update the consensus statement issued in 1989 by a panel of neurologists and metabolic experts on L-carnitine supplementation in childhood epilepsy. The panelists agreed that intravenous L-carnitine supplementation is clearly indicated for valproate (VPA)-induced hepatotoxicity, overdose, and other acute metabolic crises associated with carnitine deficiency. Oral supplementation is clearly indicated for the primary plasmalemmal carnitine transporter defect. The panelists concurred that oral L-carnitine supplementation is strongly suggested for the following groups as well: patients with certain secondary carnitine-deficiency syndromes, symptomatic VPA-associated hyperammonemia, multiple risk factors for VPA hepatotoxicity, or renal-associated syndromes; infants and young children taking VPA; patients with epilepsy using the ketogenic diet who have hypocarnitinemia; patients receiving dialysis; and premature infants who are receiving total parenteral nutrition. The panel recommended an oral L-carnitine dosage of 100 mg/kg/day, up to a maximum of 2 g/day. Intravenous supplementation for medical emergency situations usually exceeds this recommended dosage.
Elmazar MM, Nau H. Ethanol potentiates valproic acid-induced neural tube defects (NTDs) in mice due to toxicokinetic interactions.
Reprod Toxicol 1995 Sep-Oct;9(5):427-433.
Abstract: Both the antiepileptic drug valproic acid (VPA) and ethanol interfere with fetal folate metabolism, which may contribute to their mechanism of teratogenesis. Therefore, the possible interaction between VPA and ethanol was investigated in mice. Ethanol (2 x 2.5 g/kg) was given orally 4 and 1 h prior to VPA (300 and 400 mg/kg, SC) in day 8.25 pregnant NMRI mice. Fetuses were examined for exencephaly, embryolethality, and fetal weight retardation on day 18 of gestation. Higher doses of ethanol (2 x 5 g/kg, orally) at day 7.5 and 8 of gestation resulted in 22% embryolethality and 1.7% exencephaly with no effect on fetal weight. Ethanol, however, increased VPA (400 mg/kg, SC)-induced exencephaly, embryolethality, and fetal weight retardation. It also increased VPA (300 mg/kg, SC)-induced exencephaly without affecting embryotoxicity. A minimum of two oral doses of 2.5 g/kg ethanol, 1 and 4 h, or 1 and 6 h prior to VPA administration were needed to produce maximum potentiation of the effects observed. These ethanol doses increased plasma VPA levels of day 8.25 pregnant mice given 400 mg/kg VPA to values comparable to the levels of mice given only VPA at a higher dose level (500 mg/kg). The incidence of exencephaly was increased from 35% for VPA (400 mg/kg) to 59% when VPA was given with ethanol. This incidence was similar to that of 60% for the high dose of VPA (500 mg/kg) administered without ethanol. Maternal plasma ethanol concentration peaked at 193, 196, and 183 mg/dL 15, 30, and 60 min, respectively, after oral ethanol administration (2.5 g/kg), and fell to 110 mg/dL by 2 h.
Freeman JM, Vining EPG, Cost S, Singhi P. Does carnitine administration improve the symptoms attributed to anticonvulsant medications? A double-blinded, crossover study.
Pediatr 1994 Jun;93(6 Pt 1):893-895.
Abstract: OBJECTIVE. This study was designed to assess the reported improvement in "well-being" perceived by parents when children who are taking anticonvulsant medications are administered carnitine. METHODOLOGY. Forty-seven children with seizures who were taking either valproic acid or carbamazepine were enrolled in a placebo-controlled, double-blinded, cross-over study of the effects of oral carnitine administration (100 mg/kilo) on their well-being as perceived by their parents. The well-being scores were assessed weekly by phone and in person at the start and end of each 4-week phase. RESULTS. The children's well-being scores improved weekly when either placebo or carnitine were administered. None of the analyses of improved well-being achieved statistical significance. CONCLUSION. We believe this study documents the necessity for controlled trials when assessing the subjective, beneficial effects of medications. Carnitine is expensive, costing approximately $.30/kilogram of body weight per day ($6 per day for a 20 kilo child). It would not appear warranted to administer carnitine prophylactically to children on anticonvulsant medications for alleviating common, nonspecific symptoms. Because there are no reliable clinical or laboratory tests of symptomatic carnitine deficiency caused by anticonvulsant administration, how to identify children in need of carnitine, and when to administer carnitine therapeutically to children receiving valproate or other anticonvulsants is unclear.
Gidal BE, Inglese CM, Meyer JF, et al. Diet-and valproate-induced transient hyperammonemia: Effect of L-carnitine.
Pediatr Neurol 1997 May;16(4):301-305.
Abstract: Hyperammonemia is an adverse effect of valproate (VPA) treatment. In particular, transient hyperammonemia has been reported to occur in VPA-treated patients after protein-rich meals. This phenomenon may occur secondary to a VPA-mediated carnitine insufficiency. We sought to confirm that protein ingestion would result in transient hyperammonemia and to determine whether supplementation with L-carnitine would prevent this effect. We studied the effect of consumption of a standardized protein-rich meal (45 g protein) before (phase I) and after (phase II) administration of L-carnitine 50 mg/kg/day for 7 days in 11 epileptic children (13.3 +/- 2.3 years of age) receiving VPA. Venous blood was obtained during fasting (baseline) and at 2 and 4 hours after the protein-rich meal for analysis of ammonia (NH3), and VPA concentrations. Mean VPA trough concentrations did not differ significantly at any time. After protein ingestion, 2-hour NH3 concentration increased by 86% (P < .05) from baseline in phase I as compared with a 38% increase in phase II. In both phases I and II, 4-hour NH3 concentrations decreased toward baseline values. We conclude that (1) modest protein ingestion can result in significant transient increases in NH3 in VPA-treated children, (2) significant increases may occur in patients with normal fasting NH3 concentrations, (3) these increases can be significantly attenuated by L-carnitine supplementation, and (4) these changes do not appear to be related to changes in VPA concentration.
Goggin T, Gough H, Bissessar A, Crowley M, Baker M, Callaghan N. A comparative study of the relative effects of anticonvulsant drugs and dietary folate on the red cell folate status of patients with epilepsy.
Q J Med 1987 Nov;65(247):911-919.
Graf WD, Oleinik OE, Glauser TA, Maertens P, Eder DN, Pippenger CE. Altered antioxidant enzyme activities in children with a serious adverse experience related to valproic acid therapy.
Neuropediatrics 1998 Aug;29(4):195-201.
Abstract: Specific oxidative metabolites of valproic acid (VPA) have been associated with the clinically defined toxicity of the drug. To investigate the role of enzymatic detoxification in clinical toxicity, we compared activities of five antioxidant enzymes in 15 patients with a serious adverse experience (SAE) related to VPA therapy, to enzyme activities measured in 35 patients with good clinical tolerance of VPA, and 50 healthy, age-matched subjects. These enzymes included glutathione peroxidase (GSH-Px), glutathione reductase (GSSG-R), glutathione transferase, superoxide dismutase, and catalase in erythrocytes; and GSH-Px in plasma. We also determined levels of Se, Cu, and Zn, trace elemental cofactors for these enzymes, in plasma from each individual. In patients with a VPA-associated SAE, GSH-Px was significantly depressed and GSSG-R was significantly elevated relative to values for the other groups. Selenium and zinc concentrations were lower in SAE patients than in controls. These findings may indicate a role for selenium dependent antioxidant activity in individual susceptibility to an SAE related to VPA therapy.
Hansen DK, Grafton TF, Dial SL, Gehring TA, Siitonen PH. Effect of supplemental folic acid on valproic acid-induced embryotoxicity and tissue zinc levels in vivo.
Teratology 1995 Nov;52(5):277-285.
Abstract: Valproic acid (VPA) is an anti-convulsant drug known to cause spina bifida in humans. Administration of the vitamin, folic acid, has been shown to decrease the recurrence and possibly also the occurrence of neural tube defects, primarily spina bifida, in humans. Additionally, treatment with a derivative (folinic acid) of folic acid has been reported to decrease the frequency of VPA-induced exencephaly in mice treated with the drug in vivo. A protective effect by folinic acid has not been observed in vitro. The purpose of this investigation was to reexamine the ability of folinic acid to decrease the incidence of VPA-induced neural tube defects in vivo. We also examined the effect of increased intake of folic acid on zinc levels in various maternal and embryonic tissues. Folinic acid, whether administered by intraperitoneal injection or in osmotic mini-pumps, did not decrease the number of mouse fetuses with VPA-induced exencephaly. Dietary supplementation with 10-20 times the daily required intake of folic acid in rodents also failed to decrease the embryotoxicity of VPA. Such dietary supplementation had no effect on zinc levels in maternal liver, brain, or kidney, nor in embryonic tissues. These results indicate that folic acid is not able to reverse the embryotoxicity induced by the anticonvulsant, that there is no apparent effect of high dietary folate intake on maternal or embryonic zinc levels and suggest that folate is probably not involved in the mechanism of VPA-induced embryotoxicity.
Hendel J, Dam M, Gram L, Winkel P, Jorgensen I. The effects of carbamazepine and valproate on folate metabolism in man.
Acta Neurol Scand 1984 Apr;69(4):226-231.
Abstract: The effect of carbamazepine and valproate treatment on folate metabolism was studied in 11 epileptic patients. The absorption of folic acid and of Pteroyl-gamma-L-glutamyl-gamma-L-glutamyl-L-glutamic acid, a synthetic substrate for intestinal folate deconjugase, was measured prior to and after 2 months of antiepileptic therapy with either carbamazepine (5 cases) or valproate (6 cases). After 2 months' treatment, the area under plasma concentration versus time curve was significantly decreased and t-max (time when maximal plasma concentration is obtained) was significantly prolonged. No inhibition of intestinal folate deconjugation was observed and the liver metabolism of folic acid was found to be unaffected by the treatment. These findings are interpreted as an inhibition of intestinal folic acid absorption caused by the antiepileptic therapy.
Hiraoka A, Arato T, Tominaga I. Reduction in blood free carnitine levels in association with changes in sodium valproate (VPA) disposition in epileptic patients treated with VPA and other anti-epileptic drugs.
Biol Pharm Bull 1997 Jan;20(1):91-93.
Abstract: Reduction in the blood free carnitine (FC) level as a side effect of sodium valproate (VPA) given epileptic patients was pharmacokinetically studied in connection with changes in the VPA disposition. The serum FC level in patients taking at least one of phenobarbital (PB), phenytoin (PHT) and/or carbamazepine (CBZ) in addition to VPA was significantly lower than that in the controls given only these other anti-epileptic drugs (AEDs). Patients medicated only with VPA also tended to have a lower serum FC level than the controls, although the difference was not significant. Among all the patients taking VPA with or without other AED(s), a significantly positive correlation was observed between the serum FC level and the value of dose and level ratio (L/D) of VPA, indicating that both the serum FC concentration and the L/D value of VPA were remarkably reduced in those patients receiving both medications. These results suggested that reduction in the blood FC level as a side effect of VPA reflected FC deficiency associated with the accelerated degradation of VPA in liver; such a condition appears to result from medication with VPA and other AED(s) which induce(s) enzyme(s) for the VPA metabolism.
Hirose S, Mitsudome A, Yasumoto S, Ogawa A, Muta Y, Tomoda Y. Valproate therapy does not deplete carnitine levels in otherwise healthy children.
Pediatrics 1998 May;101(5):E9.
Abstract: OBJECTIVE: To determine whether children with epilepsy undergoing valproate (VPA) antiepileptic therapy and who are otherwise healthy have a lower serum level of carnitine (CAR) and a higher plasma level of plasma ammonia than do normal children. METHODOLOGY: A total of 45 children with epilepsy, 6.3 to 21.7 years of age, who were treated solely with VPA and were free of abnormal neurologic findings or nutritional problems were randomly selected (VPA-treated group). An age-matched control group (n = 45) was selected from subjects without epilepsy (control group). Total (T) and free (F) serum CAR, serum VPA concentration, and the plasma ammonia level were measured and analyzed. RESULTS: Serum VPA concentration exhibited a weak negative correlation with both T- (r = -0.34) and F-CAR (r = -0.41). The T-CAR levels were 55.7 +/- 12.4 and 57.6 +/- 12.1 mM, and the F-CAR levels 42.7 +/- 9.9 and 44.4 +/- 9.9 mM in the VPA-treated and control groups, respectively. Thus, there was no significant difference in T- or F-CAR levels between the VPA-treated and control groups. Plasma ammonia levels were the same in the two groups: 26 +/- 9.2 and 29.4 +/- 11.8 mM in the VPA-treated and control groups, respectively. There was no significant correlation between blood ammonia and either T- (r = +0.024) or F-CAR (r = -0. 026). CONCLUSION: Children on a regular diet ingest a sufficient amount of CAR that more than meets their daily CAR requirement. The level of neither T- nor F-CAR in patients with epilepsy and without severe neurologic or nutritional problems being treated with VPA appeared to be affected by VPA therapy. Because the blood CAR level depends on nutritional condition rather than on blood VPA concentration, CAR deficiency caused by VPA is not likely to occur in this population. The usefulness of supplementation of CAR for this type of patient with epilepsy, therefore, must be reevaluated carefully.
Hurd RW, Van Rinsvelt HA, Wilder BJ, Karas B, Maenhaut W, De Reu L. Selenium, zinc, and copper changes with valproic acid: possible relation to drug side effects.
Neurology 1984 Oct;34(10):1393-1395.
Abstract: Side effects of treatment with the anticonvulsant valproic acid (VPA) suggested the possibility of alteration of trace metal status. Administration of VPA for 1 week produced significant depletion of zinc and selenium in plasma of rats and a one-third reduction of hepatic selenium. Patients who were treated chronically, with VPA as the sole anticonvulsant medication, had decreased plasma selenium levels. Most cases of VPA-associated hepatotoxicity occur in children. This could be due to decreased selenium concentrations when mechanisms for protection against peroxidative damage are not fully developed.
Ito M, Okuno T, Hattori H, Fujii T, Mikawa H. Vitamin B6 and valproic acid in treatment of infantile spasms.
Pediatr Neurol 1991 Mar-Apr;7(2):91-96.
Abstract: Twenty patients with infantile spasms were treated with high doses of vitamin b6, valproic acid, or both. Three of 13 patients (23%) treated initially with high doses of vitamin B6 demonstrated a definite reduction in seizures; 2 patients had no improvement on electroencephalography. Vitamin B6 therapy alone was continued in a single patient (8%) who remained seizure-free during the 15-month follow-up period. Initial treatment with vitamin B6 and valproic acid improved the electroencephalogram significantly more (P less than 0.05) than initial vitamin B6 treatment alone. The group which had valproic acid added to vitamin B6 therapy had significantly fewer seizures (P less than 0.05) and better electroencephalograms (P less than 0.01) than did the group treated initially with vitamin B6 alone. There were no significant differences among the group treated initially with vitamin B6, the group treated initially with valproic acid, and the group in which valproic acid was substituted for vitamin B6. ACTH was more effective in abolishing seizures than was valproic acid or vitamin B6 and valproic acid. ACTH had an excellent effect on seizures in 86% of patients who did not respond well to vitamin B6, valproic acid, or both; however, many of these patients had later recurrence of infantile spasms. The combination of vitamin B6 and valproic acid is effective and safe in the treatment of infantile spasms.
Jurima-Romet M, Abbott FS, Tang W, Huang HS, Whitehouse LW. Cytotoxicity of unsaturated metabolites of valproic acid and protection by vitamins C and E in glutathione-depleted rat hepatocytes.
Toxicology 1996 Aug 1;112(1):69-85.
Abstract: Valproic acid (VPA) and the unsaturated metabolites, 2-ene VPA and (E)-2,(Z)-3'-diene VPA, demonstrated dose-dependent cytotoxicity in primary cultures of rat hepatocytes, as evaluated by lactate dehydrogenase (LDH) leakage. Cellular glutathione (GSH) was depleted by adding buthionine sulfoximine (BSO) to the culture medium. Induction of cytochrome P450 by pretreatment of rats with phenobarbital or pregnenolone-16 alpha-carbonitrile enhanced the cytotoxicity of parent VPA in BSO-treated hepatocytes. The cytotoxicity of 4-ene VPA was apparent in BSO-treated hepatocytes with detectable loss of cell viability at 1 microM of added 4-ene VPA. Depletion of cellular GSH also increased the cytotoxicities of 2-ene VPA and (E)-2,(Z)-3'-diene VPA. The cytotoxicity of 2-ene VPA was comparable to or higher than that of VPA, producing loss of viability at concentrations > or = 5 mM. Time-course evaluation of hepatocyte response to 4-ene VPA in the GSH-depleted state revealed a delayed cytotoxicity with no effect during the first 12 h of exposure followed by a pronounced toxicity between 12 and 14 h. Two major GSH conjugates of 4-ene VPA metabolites, namely 5-GS-4-hydroxy VPA lactone and 5-GS-3-ene VPA, were detected in 4-ene VPA treated hepatocytes. Consistent with this finding, a 50% decrease in cellular GSH levels was observed following 4-ene VPA treatment. Under similar conditions, neither toxicity nor the GSH conjugated metabolite were detected in cells treated with the alpha-fluorinated 4-ene VPA analogue (alpha-F-4-ene VPA). The antioxidants, vitamin C and vitamin E, demonstrated a cytoprotective effect against 4-ene VPA-induced injury in GSH-depleted hepatocytes. These results are in support of hepatocellular bioactivation of VPA via 4-ene VPA to highly reactive species, which are detoxified by GSH. The susceptibility of hepatocytes to VPA metabolite-mediated cytotoxicity depends on cellular GSH homeostasis.
Kaji M, Ito M, Okuno T, Momoi T, Sasaki H, Yamanaka C, Yorifuji T, Mikawa H. Serum copper and zinc levels in epileptic children with valproate treatment.
Epilepsia 1992 May-Jun;33(3):555-557.
Abstract: Valproate (VPA) induces zinc (Zn) deficiency in experimental animals, but whether VPA treatment induces deterioration of serum trace metal homeostasis in humans is uncertain. We measured serum copper (Cu) and Zn levels in epileptic children treated with VPA and/or other antiepileptic drugs (AEDs). Patients treated with VPA monotherapy had significantly lower levels of serum Cu (82.2 +/- 16.6 micrograms/dl) than normal controls (97.3 +/- 23.0 micrograms/dl). Patients treated with VPA in addition to some other AED also had significantly lower levels of serum Cu (84.8 +/- 20.0 micrograms/dl). Serum Cu concentrations in patients treated with AEDs except for VPA (87.7 +/- 19.1 micrograms/dl) were not statistically different from those of control subjects. In contrast to the reported results of animal experiments, serum Zn levels were not altered in patients with VPA treatment. Although none of our patients showed any symptoms of Cu deficiency, we should pay attention to potential Cu deficiency in patients with VPA treatment.
Kelley RI. The role of carnitine supplementation in valproic acid therapy.
Pediatr 1994 Jun;93(6 Pt 1):891-892. (Editorial)
Kishi T, Fujita N, Eguchi T, Ueda K. Mechanism for reduction of serum folate by antiepileptic drugs during prolonged therapy.
J Neurol Sci 1997 Jan;145(1):109-112.
Abstract: To determine whether the induction of liver enzymes by antiepileptic drugs play a major role in folate depletion, serum concentrations of folate were measured in age-matched control subjects without anemia and in epileptic outpatients who were being treated with a single antiepileptic drug. Two of the four drugs being administered were enzyme inducers. A protein binding radioassay was used to measure folate levels. Compared with serum folate levels in controls (5.14 +/- 1.88 ng/ml: n = 74), mean serum folate levels were reduced significantly in patients treated with phenobarbitone (3.91 +/- 1.73 ng/ml, p < 0.01: n = 33) and carbamazepine (3.85 +/- 1.02 ng/ml, p < 0.01: n = 36): both of which are enzyme-inducing agents. In contrast, patients treated with the non-enzyme-inducer valproate (5.39 +/- 1.71 ng/ml: n = 41) or zonisamide (5.59 +/- 2.60 ng/ml: n = 25) exhibited serum folate levels that did not differ significantly from values in controls. Findings showed that a reduction in serum folate is associated with the induction of enzymes by antiepileptic drugs. Thus, the induction of microsomal liver enzymes may be critical to the depletion of folate by antiepileptic drugs.
Kryzhanovskii GN, Shandra AA. [Effect of diazepam, carbamazepine, sodium valproate and their combinations with vitamin preparations on epileptic activity.]
Biull Eksp Biol Med 1985 Nov;100(11):545-547. [Article in Russian]
Abstract: The anticonvulsive action of diazepam, carbamazepine, sodium valproate and their combinations with pyridoxal-5-phosphate, nicotinamide, and alpha-tocopherol were investigated in acute experiments on mice with corazole-induced seizures. Diazepam (0.5 mg/kg), carbamazepine (50 mg/kg) and sodium valproate (200 mg/kg) were shown to reduce convulsive intensity and lethality. Vitamins nicotinamide (250 mg/kg), pyridoxal-5-phosphate (10 mg/kg) and alpha-tocopherol (100 mg/kg) potentiated anticonvulsive action of the above antiepileptic drugs. The results of the investigation suggest the efficacy of pathogenetic therapy and give new evidence of the advisability of using vitamins in combination with synthetic anticonvulsive drugs.
Kurekci AE, Alpay F, Tanindi S, Gokcay E, Ozcan O, Akin R, Isimer A, Sayal A. Plasma trace element, plasma glutathione peroxidase, and superoxide dismutase levels in epileptic children receiving antiepileptic drug therapy.
Epilepsia 1995 Jun;36(6):600-604.
Abstract: Some antiepileptic drugs (AEDs) may alter trace element metabolism and free radical scavenging enzyme activities in humans and experimental animals. We investigated the effect of long-term AED therapy on copper (Cu), zinc (Zn), manganese (Mn), selenium (Se), magnesium (Mg), glutathione peroxidase (GSH-PX), and superoxide dismutase (SOD) in the plasma in children with epilepsy. During treatment with valproate (VPA) or carbamazepine (CBZ) monotherapy plasma Cu, Zn, Mn, Se, and Mg concentrations of patients were not statistically different from those of control subjects. The level of serum VPA weakly correlated with the increase in plasma Zn level. Recent studies suggest that membrane lipid peroxidation may be causally involved in some forms of epilepsies, and the decreased free radical scavenging enzyme activity is believed to cause the increased risk of an idiosyncratic drug reaction encountered in the management of epilepsy. Because GSH-PX and SOD are the most important members of antioxidant defense mechanisms, we quantitated the activities of these enzymes in plasma of children with epilepsy receiving VPA or CBZ. Only plasma GSH-PX activities in VPA group were higher than those of the control group, and the difference was statistically significant
Lerman-Sagie T, Statter M, Szabo G, Lerman P. Effect of valproic acid therapy on zinc metabolism in children with primary epilepsy.
Clin Neuropharmacol 1987;10(1):80-86.
Abstract: The effect of long-term treatment with valproic acid (VPA) on zinc (Zn) metabolism was studied in 15 children with absence seizures. During treatment with VPA the erythrocyte Zn content was significantly lower than that found in controls matched for sex and age. Plasma and urine values of Zn and of copper were within normal limits. It is suggested that the anticonvulsive action of VPA may be mediated through its effect on the metabolism of Zn in the brain and the concomitant changes in the activity of the enzymes glutamic acid decarboxylase and carbonic anhydrase.
Murakami K, Sugimoto T, Woo M, Nishida N, Muro H. Effect of L-carnitine supplementation on acute valproate intoxication.
Epilepsia 1996 Jul;37(7):687-689.
Abstract: We analyzed urinary valproate (VPA) metabolites and carnitine concentrations in a child who accidentally ingested 400 mg/kg VPA. The concentration of 4-en VPA, the presumed major factor in VPA-induced hepatotoxicity, was markedly increased, without liver dysfunction or hyperammonemia. The other major abnormality was decreased beta-oxidation and markedly increased omega-oxidation. After L-carnitine supplementation, VPA metabolism returned to normal. The level of valproylcarnitine was not increased and therefore was not affected by L-carnitine. L-Carnitine may be useful in treating patients with coma after VPA overdose.
Navarro-Quesada FJ, Lluch-Fernandez MD, Vaquero-Abellan M, Marchante-Serrano C, Jimenez C. [Evaluation of the effect of long term valproic acid treatment on plasma levels of carnitine, ammonia and amino acids related to the urea cycle in pediatric epileptic patients]. Rev Neurol 1997 Jul;25(143):1037-1044. [Article in Spanish]
Abstract: INTRODUCTION: Valproic acid (VPA) is an antiepileptic drug widely used in paediatrics. In spite of being a safe and effective anticonvulsant, VPA has been involved in the onset of changes in the metabolism of ammonia and carnitine, although few prospective studies have been made of this. OBJECTIVES: To evaluate the effect of long-term VPA administration, particularly on the metabolism of carnitine, ammonia and plasma amino-acids and the possible clinical repercussions of this in a group of epileptic patients studied prospectively and retrospectively. MATERIAL AND METHODS: A study was made of 102 epileptic children on long term anticonvulsant treatment mainly with VPA. These patients were divided into two groups: group I (n = 25) were studied prospectively (basal sample, after one, six and twelve months of treatment) and group II (n = 77) or long term treatment group (a single sample extraction). In each epileptic patient and in 56 children from a control group (group III) studies were made of free plasma carnitine, ammonia and amino-acids related to the urea cycle and the plasma levels of each anticonvulsant drug. RESULTS: It was observed that in group I there was a fall in plasma carnitine concentrations with time and a progressive rise which was statistically significant (p = 0.001) in plasma levels, mainly of ammonia, glutamine, glycine and ornithine, from the basal levels to those after a year of treatment in practically 100% of the children studied. In group II children on antiepileptic drugs, mainly VPA, were seen to have lower plasma carnitine levels than those in the control group and higher serum ammonia, glutamine and glycine levels than the healthy population not treated with anticonvulsants. These differences were statistically significant (p = 0.001). No relationship was found between the parameters studied and the plasma levels of the drug, type of epilepsy or presence of side effects. CONCLUSIONS: These changes show the negative effects of VPA on the metabolism of carnitine and ammonia. It would therefore seem advisable to monitor these parameters in epileptic children on long term antiepileptic treatment.
Nurge ME, Anderson CR, Bates E. Metabolic and nutritional implications of valproic acid.
Nutr Res 1991;11:949-960.
Pietz J, Benninger C, Schafer H, Sontheimer D, Mittermaier G, Rating D. Treatment of infantile spasms with high-dosage vitamin B6.
Epilepsia 1993 Jul-Aug;34(4):757-763.
Abstract: High-dose vitamin B6 (pyridoxine-HCl, 300 mg/kg/day orally) was introduced as the initial treatment of recently manifested infantile spasms in 17 children (13 symptomatic cases with identified brain lesion and 4 cryptogenic cases). 5 of 17 children (2 cryptogenic, 2 with severe pre/perinatal brain damage and one with Sturge-Weber syndrome) were classified as responders to high-dose vitamin B6. In all 5 cases the response to vitamin B6 occurred within the first 2 weeks of treatment and within 4 weeks all patients were free of seizures. Two patients developed other seizures (partial seizures, etiologically unclear blinking attacks), but no relapse of infantile spasms was observed among the five responders to vitamin B6. No serious adverse reactions were noted. Side effects were mainly gastrointestinal symptoms, which were reversible after reduction of the dosage. Considering the life-threatening side effects of treatment with ACTH/corticosteroids or valproate, a controlled clinical trial with high-dose vitamin B6 would appear justified to either prove or disprove efficacy.
Raby WN. Carnitine for valproic acid-induced hyperammonemia. Am J Psychiatry. 1997 Aug;154(8):1168-1169.
Roe DA. Drug-induced Nutritional Deficiencies. 2nd Edition. Westport, CT: Avi Publishing, 1985: 245-259.
Sakemi K, Takada G. Effect of carnitine on valproic acid concentrations in serum, brain, and liver.
Pediatr Neurol 1998 Apr;18(4):331-333.
Abstract: This study investigates the influence of L-carnitine supplementation on valproic acid concentrations in rat serum, brain, and liver. Carnitine supplementation increased carnitine concentrations significantly in serum and liver but not in the brain. Free valproic acid concentrations in the brain were significantly increased by carnitine supplementation without any change of carnitine concentrations in the brain. The increase of serum-free valproic acid concentrations by carnitine supplementation apparently caused brain-free valproic acid concentrations to increase. This study suggests that L-carnitine supplementation to valproic acid therapy may potentiate valproic acid effects in the brain, even when the clinical dosage in humans is used.
Schwaninger M, Ringleb P, Winter R, Kohl B, Fiehn W, Rieser PA, Walter-Sack I. Elevated plasma concentrations of homocysteine in antiepileptic drug treatment.
Epilepsia 1999 Mar;40(3):345-350.
Abstract: PURPOSE: Homocysteine is an experimental convulsant and an established risk factor in atherosclerosis. A nutritional deficiency of vitamin B6, vitamin B12, or folate leads to increased homocysteine plasma concentrations. During treatment with carbamazepine (CBZ), phenytoin, or phenobarbital, a deficiency in these vitamins is common. The objective of the study was to test the hypothesis that antiepileptic drug (AED) treatment is associated with increased homocysteine plasma concentrations. METHODS: A total of 51 consecutive outpatients of our epilepsy clinic receiving stable, individually adjusted AED treatment and 51 sex- and age-matched controls were enrolled in the study. Concentrations of total homocysteine and vitamin B6 were measured in plasma; vitamin B12 and folate were measured in the serum of fasted subjects. RESULTS: Patients and controls differed significantly in concentrations of folate ( 13.5+/-1.0 vs. 17.4+/-0.8 nM and vitamin B6 (39.7+/-3.4 vs. 66.2+/-7.5 nM), whereas serum concentrations of vitamin B12 were similar. The homocysteine plasma concentration was significantly increased to 14.7+/-3.0 microM in patients compared with controls (9.5+/-0.5 microM; p < 0.05, Wilcoxon rank-sum test). The number of patients with concentrations of >15 microM was significantly higher in the patient group than among controls. The same result was obtained if only patients with CBZ monotherapy were included. Patients with increased homocysteine plasma concentrations had lower folate concentrations. CONCLUSIONS: These data support the hypothesis that prolonged AED treatment may increase plasma concentrations of homocysteine, although the alternative explanation that increased homocysteine plasma concentrations are associated with the disease and not the treatment cannot be completely excluded at the moment.
Sozuer DT, Barutcu UB, Karakoc Y, Yalcin E, Onen S. The effects of antiepileptic drugs on serum zinc and copper levels in children.
J Basic Clin Physiol Pharmacol 1995;6(3-4):265-269.
Abstract: The results of previous studies that examined serum trace metal status of epileptic patients receiving antiepileptic drug (AED) therapy were variable. We measured serum zinc (Zn) and copper (Cu) levels in 52 epileptic children who were treated with either carbamazepine (CBZ) or valproic acid (VPA) or with a combination of CBZ and VPA. Serum Zn levels were significantly lower in the epileptics than in the two control groups which consisted of 7 untreated epileptics and 12 normal children (p < 0.05). Combination therapy and monotherapy with CBZ increased serum Cu levels (p < 0.05). No significant alteration in serum Cu levels was observed with VPA monotherapy. Serum Zn and serum Cu concentrations of the untreated epileptics were not significantly different from those of normal controls. Our results indicate that serum trace metal homeostasis may be affected by AED therapy, but not by the convulsive disorder itself.
Stadler DD, Bale JF Jr, Chenard CA, Rebouche CJ. Effect of long-term valproic acid administration on the efficiency of carnitine reabsorption in humans.
Metabolism 1999 Jan;48(1):74-79.
Abstract: To elucidate the etiology of valproic acid-induced carnitine deficiency, we tested the hypothesis that long-term valproic acid administration decreases the rate of carnitine reabsorption. Thirteen healthy men participated in a 34-day protocol in which carnitine clearance was measured before and after 28 days of valproic acid administration. During valproic acid administration (days 6 to 33), plasma free and total carnitine concentrations decreased (18% and 12%, respectively, P<.05) by 16 days, but returned to pretreatment concentrations by 28 days. From day 14 to day 30, the rate of free carnitine excretion was 50% lower than at baseline (day 4, P<.05). Free and total carnitine clearance, indexed to the glomerular filtration rate, was lower after valproic acid administration (P<.01). Contrary to our hypothesis, after 28 days of valproic acid administration, the rate of carnitine reabsorption was enhanced independent of the glomerular filtration rate and filtered load. Changes in the plasma concentration, rate of excretion, and clearance were specific for carnitine and were not generalized in magnitude or direction to the other amino acids. We conclude that the kidney adapts to conserve carnitine during valproic acid administration and therefore does not cause valproic acid-induced carnitine depletion in adults.
Suzuki T, Koizumi J, Moroji T, Shiraishi H, Hori T, Baba A, Kawai N, Tada K. Effects of long-term anticonvulsant therapy on copper, zinc, and magnesium in hair and serum of epileptics.
Biol Psychiatry 1992 Mar 15;31(6):571-581.
Tabatabaei AR, Abbott FS. Assessing the mechanism of metabolism-dependent valproic acid-induced in vitro cytotoxicity.
Chem Res Toxicol 1999 Apr;12(4):323-330.
Abstract: This study was designed to distinguish and evaluate the contribution of reactive metabolite and reactive oxygen species as the mechanism of metabolism-dependent valproic acid-induced in vitro cytotoxicity. The involvement of reactive oxygen species in the mechanism of in vitro cytotoxicity was examined by the addition of a series of antioxidant enzymes and iron chelators to the reaction mixture. Addition of catalase to the reaction mixture resulted in a complete prevention of valproic acid-induced cytotoxicity. Co-incubation of a cell impermeable iron chelator deferoxamine did not effect cytotoxicity, whereas 1,10-phenanthroline, a chelator with the ability to traverse cell membranes at low concentrations, afforded significant protection against valproic acid-induced cytotoxicity. A possible inhibitory effect of catalase and 1,10-phenanthroline on the microsomal metabolism of valproic acid was disproved by the quantification of valproic acid metabolites in the presence and absence of these compounds. To assess the specificity of the mechanism of in vitro valproic acid-induced cytotoxicity, prevention of in vitro acetaminophen-induced cytotoxicity by antioxidant enzymes and iron chelators was also evaluated. Addition of catalase to the reaction mixture in the presence of acetaminophen resulted in a moderate reduction in the level of but a lack of complete protection of cytotoxicity. Addition of 1,10-phenanthroline to the reaction mixture in the presence of acetaminophen did not result in a detectable change in acetaminophen-induced cytotoxicity. These data suggest the involvement of reactive oxygen species in the mechanism of toxicity of valproic acid and perhaps reactive metabolites as the major cause of cytotoxicity in the case of acetaminophen in the in vitro model investigated. Inhibition of poly(ADP-ribose) polymerase activity by various antagonists resulted in complete prevention of valproic acid-induced in vitro cytotoxicity. The cytoprotective effects of known poly(ADP-ribose) polymerase antagonists implicate poly(ADP-ribose) polymerase in the mechanism of in vitro metabolism-dependent valproic acid-induced cytotoxicity under these conditions. These results further point to nuclear DNA as the intracellular site of insult by the generated oxygen radicals. Overall, the data obtained support the hypothesis that the metabolism-dependent valproic acid-induced in vitro cytotoxicity is the result of generation of hydrogen peroxide in the medium that can readily cross cell membranes and subsequently interact intracellularly with iron to produce the highly reactive hydroxyl free radicals.
Threlkeld DS, ed. Central Nervous System Drugs, Anticonvulsants, Valproic Acid and Derivatives. In:
Facts and Comparisons Drug Information. St. Louis, MO: Facts and Comparisons, May 1997.
Triggs WJ, Gilmore RL, Millington DS, Cibula J, Bunch TS, Harman E. Valproate-associated carnitine deficiency and malignant cerebral edema in the absence of hepatic failure.
Int J Clin Pharmacol Ther 1997 Sep;35(9):353-356.
Van Wouwe JP. Carnitine deficiency during valproic acid treatment. Internat J Vit Nutr Res
1995;65(3):211-214.
Abstract: Prolonged valproic acid treatment results in secondary carnitine deficiency. In thirteen children paired samples of plasma were drawn at the onset of, and after 9 months of continuous valproic acid treatment. At onset free plasma carnitine values were age dependent; they increased during childhood (r = 0.59, p = 0.016). After 9 months: 1 - mean plasma free carnitine decreased by 40%, from 32.7 mumol/l to 20.9 (p 0.0008 and 3% overlap; 2 - plasma total carnitine decreased by 20%, from 34.9 mumol/l to 27.1 (p 0.016 and no overlap); and 3 - the esterified/free carnitine ratio increased by 40%, from 0.28 to 0.39 (p 0.011 and no overlap). In two out of thirteen patients clinical symptoms were observed, fatigue, besides the biochemical evidence of carnitine deficiency. In four others only biochemical deficiency was found. If the child complains of fatigue during prolonged valproic acid treatment, it is advised to supplement carnitine. A dose of 15 mg/kg body weight is effective to reverse the clinical symptoms of carnitine deficiency within a week. The dose to prevent deficiency is not yet established.
Zelnik N, Fridkis I, Gruener N. Reduced carnitine and antiepileptic drugs: cause relationship or co-existence?
Acta Paediatr 1995 Jan;84(1):93-95.
Abstract: Serum carnitine was measured longitudinally before and after therapy in 15 patients receiving valproic acid, 14 patients receiving carbamazepine and 8 patients receiving phenobarbital. The patients who received valproic acid showed a significant reduction in free (and total) serum carnitine (mean (SE) 37.6 (6.2) mumol/l without valproic acid, 29.1 (1.6) mumol/l with valproic acid (p < 0.001)). Such an effect was not found in patients receiving carbamazepine or phenobarbital.

